August 2018 – A New Species of Gorgonian Octocoral from the Mesophotic Zone
PROCEEDINGS OF THE CALIFORNIA ACADEMY OF SCIENCES
A New Species of Gorgonian Octocoral from the Mesophotic Zone off the Central Coast of California, Eastern Pacific
with a Key to Related Regional Taxa(Anthozoa, Octocorallia, Alcyonacea)
Gary C. Williams 1, 4 and Odalisca Breedy 2, 3
A New Species of Gorgonian Octocoral from the Mesophotic Zone off the Central Coast of California, Eastern Pacific
with a Key to Related Regional Taxa(Anthozoa, Octocorallia, Alcyonacea)
Gary C. Williams 1, 4 and Odalisca Breedy 2, 3
1 Department of Invertebrate Zoology and Geology, California Academy of Sciences, Golden
Gate Park, 55 Music Concourse Drive, San Francisco, California 94118, USA.; 2 Centro de
Investigación en Estructuras Microscópicas, Centro de Investigación en Ciencias del Mar y Lim-
nología, Escuela de Biología, Universidad de Costa Rica. P.O. Box 11501-2060, San José, Costa
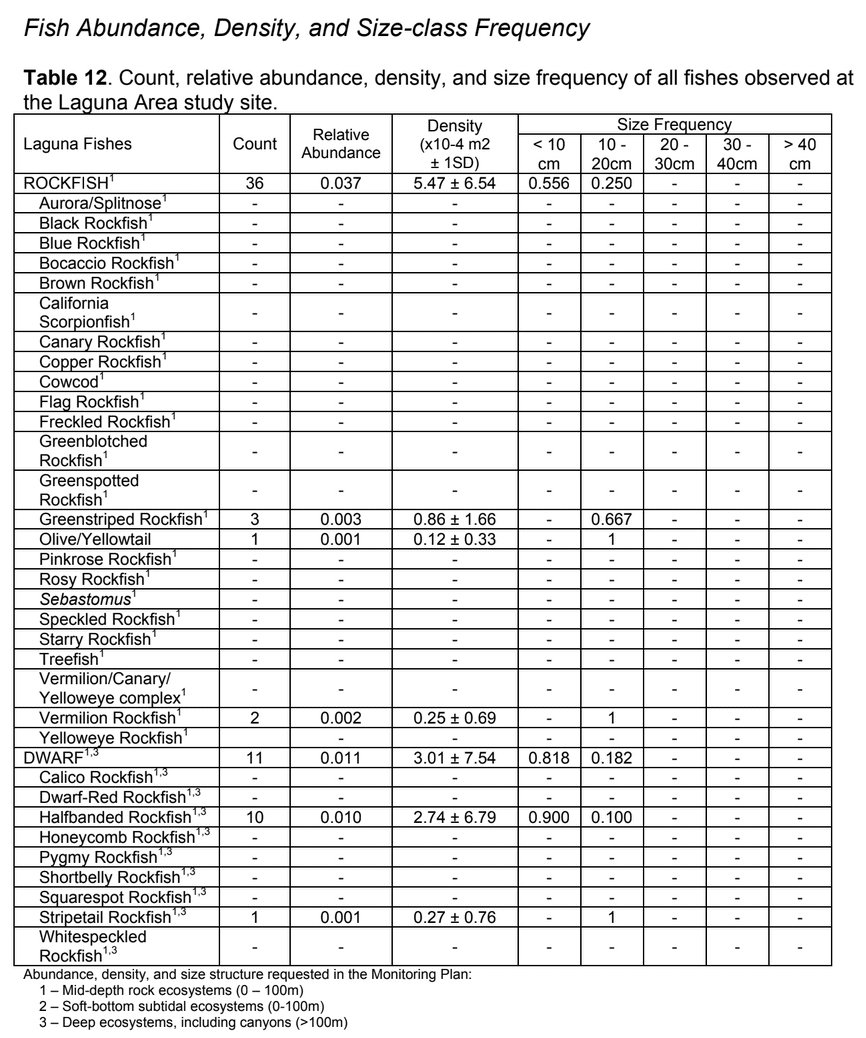
Rica; 3 Smithsonian Tropical Research Institute, P.O. Box 0843-03092, Republic of Panama;
4 Corresponding Author: Gary C. Williams (gwilliams@calacademy.org)
Recent offshore benthic surveys utilizing Remotely Operated Vehicles in the Nation- al Marine Sanctuaries along the California coastline under the auspices of the National Oceanic and Atmospheric Administration and the Ocean Exploration Trust, have yielded newly collected material and imagery of octocoral cnidarians from mesophotic and deep-sea habitats. As part of this effort, a new species of gorgonian coral is here described that was first observed at Cordell Bank, approximately 112 km WNW of San Francisco. The species is allocated to the gorgonian genus Chromoplexaura based on morphological considerations, and has since been collected or observed from four localities in central and southern California, 86–107 m in depth.
KEYWORDS: Corals, sea fans, gorgonian octocorals, Central California, Cordell Bank, mesophotic zone, taxonomic key to the genus and related taxa.
Chromoplexaura is currently regarded as a monotypic octocoral genus (Cordeiro et al. 2018c), represented by C. marki (Kükenthal, 1913), and is distributed from central Oregon to southern California on the west coast of North America. Bathymetric distribution of this species varies from nine to at least 90 m (Williams 2013). The new species described here represents a second species of the genus and is known from central to southern California with a depth range of 86 to 106 m. The two species currently share several morphological similarities. Herein we describe a new species that was first observed, but not collected in 2007 by ROV imagery at Cortes Bank in southern California, near the border between California and Mexico. In 2017, colonies were observed (also not collected) by ROV in the Cordell Bank National Marine Sanctuary in central California. In 2018, four specimens were collected by ROV and one was recorded by benthic ROV imagery on board the National Oceanic and Atmospheric Administration (NOAA) ship FSV Bell M. Shimada, at three locations in central and southern California: Cordell Bank NMS, Monterey Bay NMS, and Channel Islands NMS.
MATERIALS AND METHODS
The type material was collected during the benthic surveys of Cordell Bank and Greater Farallones National Marine Sanctuaries on board the NOAA ship FSV Bell M. Shimada (Fig. 1), between 28 July and 11 August 2018. The holotype and paratypes of the new species are deposit- ed in the marine invertebrate collections of the Department of Invertebrate Zoology and Geology at the California Academy of Sciences in San Francisco, California. Underwater video and still imagery were taken on board the ship by NOAA and MARE staff. Images of preserved material and scanning electron micrographs were taken by the first author at the California Academy of Sciences in 2018.
Abbreviations used in the text are as follows: FSV – Fisheries Survey Vessel, MARE – Marine
Applied Research and Education; CASIZ – California Academy of Sciences Invertebrate Zoology;
CBNMS – Cordell Bank National Marine Sanctuary; MBNMS – Monterey Bay National Marine Sanctuary; CINMS – Channel Islands National Marine Sanctuary; NMS – National Marine Sanc- tuary; NOAA – National Oceanic and Atmospheric Administration; ROV – Remotely Operated Vehicle.
Depths used in the text include: Shallow-water (0–40 m); Mesophotic (40–150 m); Deep-Sea (>150 m). Material used for comparative purposes: Chromoplexaura marki; CASIZ 190436; NOAA Sample S-17; Gulf of the Farallones National Marine Sanctuary, Rittenburg Bank (37.88°N 123.32°W); 89.4 m depth; 08 October 2012; ROV Beagle (MARE) from R/V Fulmar (NOAA); three terminal branches, wet-preserved in 95% ethanol. Euplexaura sp.; CASIZ 220608; Western Pacific Ocean, Caroline Islands, Palau (7.54°N 134.47°E); 7-31 m depth; 08 December 2016; cool G.C. Williams; one partial colony, wet-preserved in 95% ethanol. Swiftia torreyi; CASIZ 220958; Cordell Bank National Marine Sanctuary (37.98°N 123.49°W); 948.82 m depth; 10 August 2017;
ROV Hercules/Argus from E/V Nautilus; one whole colony, wet-preserved in 95% ethanol. 144
PROCEEDINGS OF THE CALIFORNIA ACADEMY OF SCIENCES
Series 4, Volume 65, No. 6
FIGURE 1. The National Oceanic and Atmospheric Administration (NOAA) Fisheries Survey Vessel, FSV Bell M. Shimada, conducts fisheries and oceanographic research throughout the Pacific coast of the United States. All type speci-mens of the new coral species described herein were collected by Remote Operational Vehicle (ROV) on board this ship in2018. Photo by Gary C. Williams.
SYSTEMATIC ACCOUNT
Subclass Octocorallia Haeckel, 1866
Order Alcyonacea Lamouroux, 1812
Family Plexauridae Gray, 1859
Chromoplexaura Williams, 2013
Euplexaura Kükenthal, 1913:266; 1924:93.
Chromoplexaura Williams, 2013:17.
GENERIC DIAGNOSIS.— Growth form planar and sparse, branching lateral. Retracted polyps form low rounded protuberances, mound-like to hemispherical in shape. Polyps are present on all sides of the branches, but can be arranged biserially on some narrow terminal branches. Coen-
cenchymal sclerites are primarily robust warty spindles, somewhat ovoid in shape or approaching girdled spindles. Other sclerite types that may be present include radiates, crosses, and spindles with a median waist that approach capstans. Anthocodial sclerites are rods that are straight or curved to sinuous. Colony color red or yellow due to conspicuous color of the sclerites.
TYPE SPECIES.— Euplexaura marki Kükenthal, 1913. Chromoplexaura cordellbankensis Williams and Breedy, sp. nov. Figures 2–10.
HOLOTYPE.— CASIZ 228195; NOAA Sample SH-18-09-017; Cordell Bank, Cordell Bank National Marine Sanctuary, CBNMS Transect-127; ca. 51 km W. of Point Reyes Peninsula (38°03′ 15.465′′N 123°28′48.072′′W); 100.5 m depth; 08 August 2018; ROV Beagle (MARE) from FSV
Bell M. Shimada (NOAA); one partial specimen (missing holdfast), wet–preserved in 95% ethanol.
PARATYPES.— CASIZ 228194. NOAA Sample SH-18-09-016; Cordell Bank, Cordell Bank
National Marine Sanctuary, CBNMS Transect-127; ca. 51 km W. of Point Reyes Peninsula, California, USA (38°03′15.915′′N 123°28′49.874′′W); 101.6 m depth; 08 August 2018; ROV Beagle
(MARE) from FSV Bell M. Shimada (NOAA); one partial specimen (14 mm long branch frag- ment), wet-preserved in 95% ethanol. CASIZ 207519; La Cruz Canyon, Monterey Bay National
Marine Sanctuary; California, USA (35.7694°N 121.4475°W); 106.8 m depth; 28 October 2018; coll. by ROV on board FSV Bell M. Shimada (NOAA); one whole specimen. CASIZ 207520; Anacapa Island, Channel Islands National Marine Sanctuary; California, USA (33.992°N
119.3722°W); 86 m depth; 31 October 2018; coll. by ROV on board FSV Bell M. Shimada (NOAA); one specimen in two pieces.
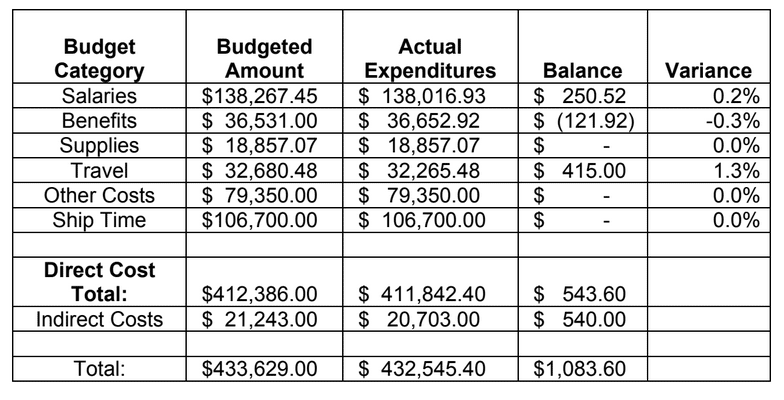
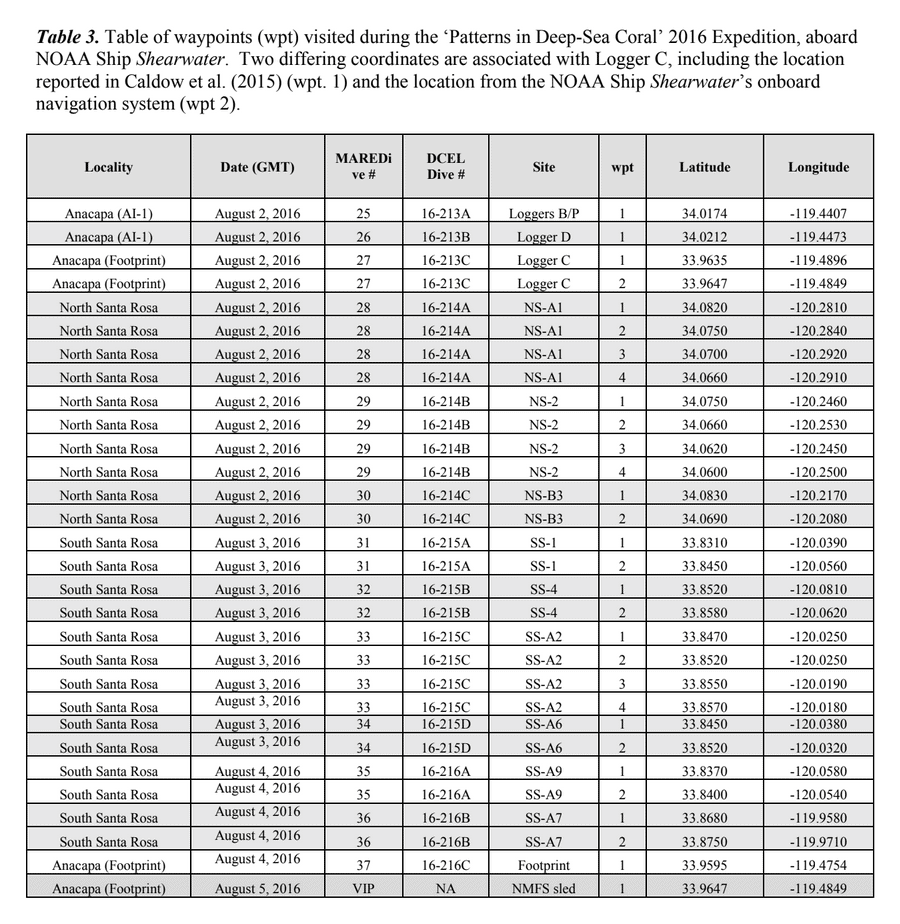
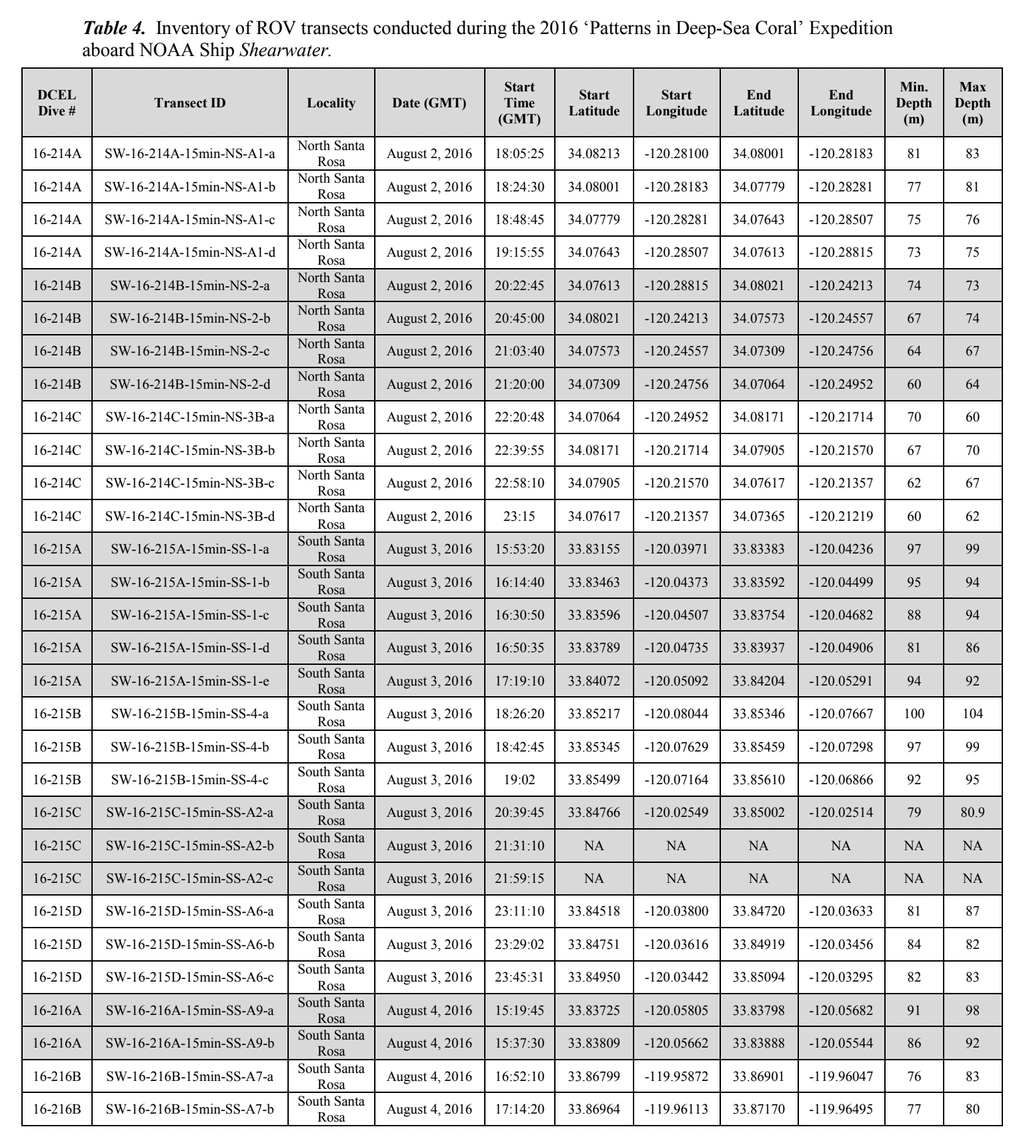
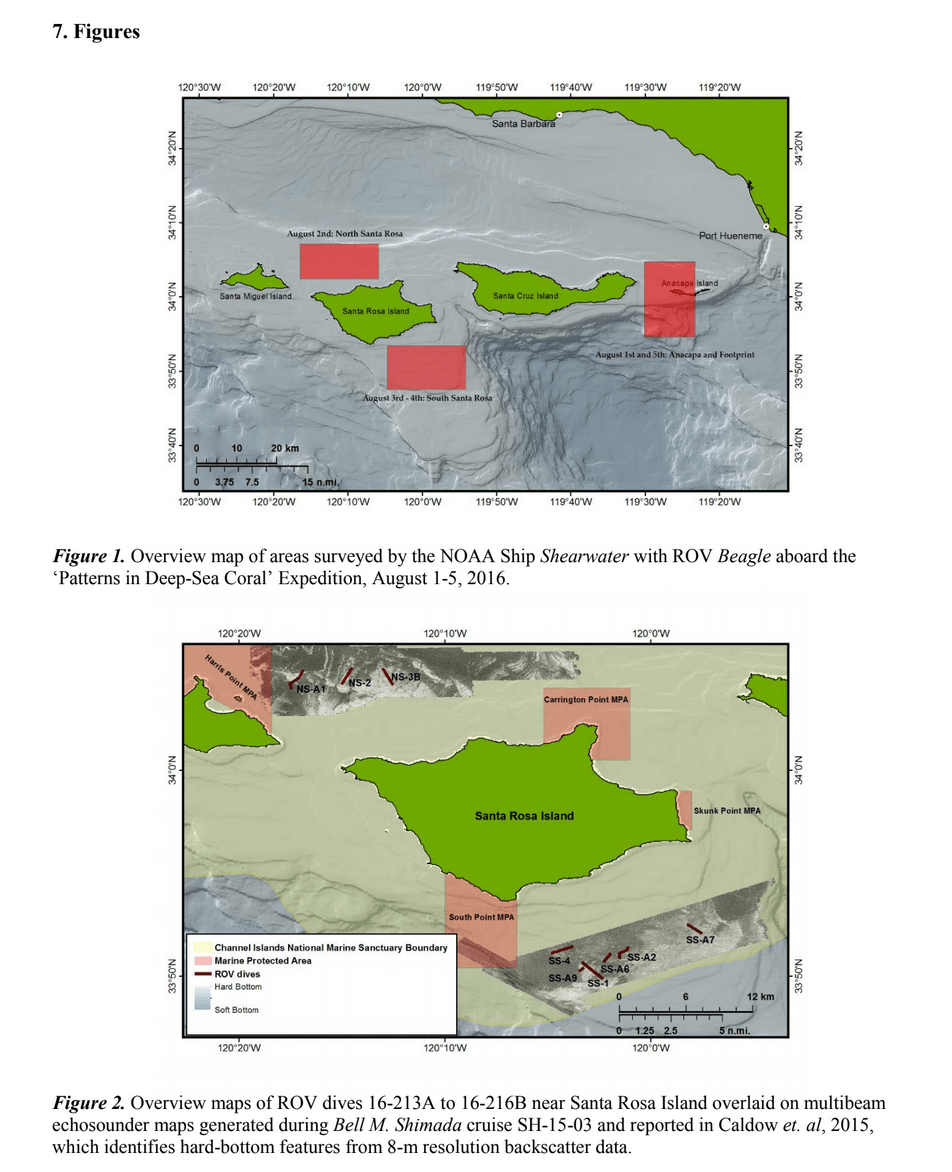
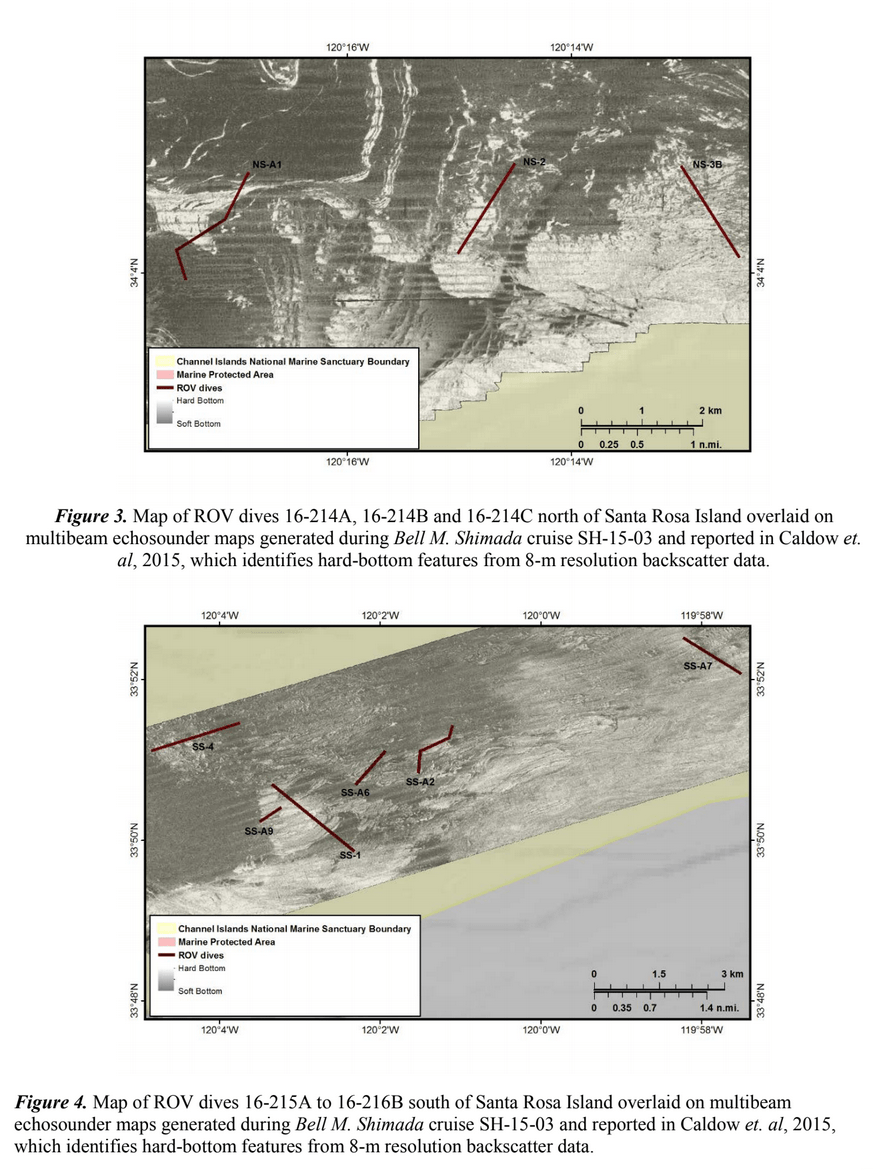
HABITAT AND DISTRIBUTION.— Found on rugose, rocky substrata often with conspicuous vertical relief, or on rounded boulders in boulder fields (Fig. 3). Distributed off the central and southern coasts of California, between 38.2° and 32.5°N latitude (Figs. 8–9); at mesophotic depths between 86 and 107 m. The type locality is Cordell Bank in the Cordell Bank National Marine Sanctuary, ca. 70 miles WNW of San Francisco, California, 100 m depth.
ETYMOLOGY.— The specific epithet is derived from Cordell Bank and the Latin suffix – ensis (belonging to); referring to the region of discovery of the new species and collection of the holotype – Cordell Bank National Marine Sanctuary.
DESCRIPTION OF THE HOLOTYPE
EXTERNAL MORPHOLOGY.— The holotype is part of a colony, 35 mm in length. The holdfast and basal portion of the colony are missing. Branching is sparse and lateral. The main stem gives rise to two lateral side branches, about 9 mm apart and 2–2.5 mm in diameter (including polyp mounds). The longest branch is 3.4 mm in length (Fig. 2). The retracted polyps form low-rounded to hemispherical polyp mounds, each < 1 mm in length. The polyps are largely distributed biserially on the thinner distal-most portions of branches (Fig. 2B), but occur all around the stouter and more basal parts of the lateral branches and main stem (Fig. 2E). There are approximately ten mounds per cm of branch length. Finger-shaped portions of the coenenchyme-covered internal axis
extend from the apical tips of some branches (Fig. 2B).
ANTHOCODIAE.— Most of the anthocodiae are preserved totally retracted into the polyp mounds, while a few are partially exserted. The walls of the anthocodiae and bases of the tentacles are relatively densely set with narrow rods that have conspicuous tuberculation (Fig. 7). Due the retracted condition of the polyps, an en chevron arrangement of sclerites was not observed or easily apparent. The sclerites of the anthocodiae are lighter in color than the coenenchymal sclerites, many appearing virtually colorless, thus resulting in a white coloration of the polyps.
The polyp mounds are represented by conspicuous rounded protuberances along the branches, usually expanded at the base while some are hemispherical in shape. Adjacent polyp mounds are generally separated by about 1.0–1.5 mm of bare rachis, and vary in width from 1.5–2.0 mm at the base, and are usually less than 1.0 mm in height (Fig. 2).
SCLERITES.— Coenenchymal sclerites vary from 0.06 to 0.22 mm in length (Figs. 4–6, 10A). They are predominantly wide, warty spindles with heavily warted tubercles, while some are narrower with less ornamentation (Figs. 4–5, 10A). Radiates and various immature forms are also present (Fig. 6). Polyp sclerites are elongate rods (Fig. 7), often slightly curved or sinuous with variable tuber- culation, while some are weakly club-shaped (Fig. 7, left). Small, flat rods (Fig. 7, center) are also present and could possibly be from the tentacles. Polyps sclerites vary in length from 0.08–0.24 mm in length.
COLOR.— Coenenchyme color is uniform lemon yellow throughout (Figs. 2–3), due to the conspicuous yellow coloration of the sclerites (Fig. 2F). The anthocodiae are colorless (Fig. 2E).
REMARKS
VARIATION: Although the holotype specimen exhibits only three branches including the main stem, the paratypes as well as additional colonies observed in underwater still images taken by ROV, all exhibit relatively sparse branching, but may possess as many as ten branches including the main stem. One of the paratype colonies (CASIZ 207519), branches up to four times and produces seven lateral branchlets.
DISCUSSION AND CONCLUSION
Key to species of Chromoplexaura and related taxa in California 1a. Colonies planar and sparsely branched. Coenenchymal sclerites are broad to ovoid spindles
with densely set tubercles, capstans, girdled spindles, elongated radiates, and/or tuberculated crosses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
1b. Colonies unbranched to copiously branched or bushy. Coenenchymal sclerites may include elongate to needlelike spindles, compact radiates, double discs, and/or disc spindles. . . . . . 3
2a. Colonies red. Coenenchymal sclerites include ovoid spindles and girdled spindles . . . . . . . . .. . . . . . . . . . . . . . . . . . . . Chromoplexaura marki (Kükenthal, 1913)
2b. Colonies yellow. Coenenchymal sclerites include capstans, elongated radiates, and crosses . .. . . . . . . . . . . . . . . . . . . Chromoplexaura cordellbankensis sp. nov.
3a. Colonies unbranched or Y-shaped. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
3b. Colonies branched – copiously branched or bushy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
4a. Colonies white; polyp mounds low-rounded. . Swiftia farallonesica Williams & Breedy, 2016
4b. Colonies coral red to dark red. Polyp mounds prominent – conical to low cylindrical. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Swiftia simplex (Nutting, 1909)
5a. Branching bushy, polyp mounds prominent – conical to cylindrical . . . . . . . . . . . . . . . . . . . . 6
5b. Branching sparse, polyp mounds low-rounded. Colonies coral red with white polyps. . . . . . . . . . . . Swiftia spauldingi (Nutting, 1909)
6a. Polyp mounds truncated conical; sclerites are radiates and elongate spindles with rounded tubercles . . . . . . . . . . . . . . . . . . . . . . Swiftia torreyi (Nutting, 1909)
6b. Polyp mounds stout, conical to cylindrical; sclerites are primarily elongate spiny spindles, often needle-like and curve . . . . . . . Swiftia kofoidi (Nutting, 1909)
TAXONOMIC ASSESSMENT
The genus Chromoplexaura is superficially similar to several Pacific coast Swiftia species. The latter is currently regarded as a gorgonian genus of twenty species (Cordeiro et al. 2018b). The type species of Swiftia is Swiftia exserta (Ellis and Solander, 1786) from the western Atlantic Ocean. Several species from the Pacific coast of the Americas have been allocated to the genus Swiftia, and it is not clear at present whether the Atlantic vs. Pacific species represent the same genus or
separate genera (Williams 2013:17). In addition, there appears to be two distinguishable groups of eastern Pacific species of Swiftia based on morphological characteristics. Preliminary molecular analyses (Everett and Park 2018; Everett, personal communication) have shown that the two
groups (Chromoplexaura and Swiftia) have not exhibited a conspicuous differentiation, but from the morphological point of view are different (Fig. 10A, B, D). An overall detailed molecular analysis and morphological comparison are necessary to provide a cogent taxonomic assessment of the relevant taxa.
Chromoplexaura cordellbankensis sp. nov. shares superficial morphological similarities with some species of Eastern Pacific Swiftia regarding external morphology – such as branching pattern, low-rounded to hemispherical polyp mounds, and elongate-tubercated anthocodial sclerites. However, the coenenchymal sclerites differ markedly from those of Swiftia, while most closely resembling the sclerite complement of Chromoplexaura marki (Williams, 2013:20–21) – i.e. the presence of robust to ovoid, highly warty spindles in the coenenchyme, which are not found in species of
Swiftia (Fig. 10A, B, D).
Chromoplexaura marki was originally placed in the Indo-Pacific genus Euplexaura by Kukenthal, 1913. However, the coenenchymal sclerites of Euplexaura species differ markedly from the two California species of Chromoplexaura, by the possession of tuberculate spheroids, subspheroids, double heads, and plump ovoid to irregular spindles (Fig. 10C; Fabricius and Alderslade 2001:190; Williams 2013:21, 24).
ACKNOWLEDGMENTS
We express our thanks to the staff scientists of NOAA (National Oceanic and Atmospheric Administration), for their support, in particular — Dan Howard and Danielle Lipski (Cordell Bank National Marine Sanctuary), Jan Roletto (Greater Farallones National Marine Sanctuary), Enrique WILLIAMS & BREEDY: NEW SPECIES OF GORGONIAN OCTOCORAL Salgado (NCCOS, National Centers for Coastal Ocean Science), and Meredith Everett (Northwest
Fisheries Science Center). We are grateful to Guy Cochrane (USGS, United States Geological Survey), Kirsten Lindquist (Gulf of the Farallones Association), the technical staff of MARE (Marine Applied Research and Exploration) — Dirk Rosen, Andy Lauermann, Heidi Lovig, Rick Botman, and Steve Holz, as well as the Marine Operations staff and crew of the NOAA ship FSV Bell M. Shimada.
LITERATURE CITED
BAYER, F.M. 1956. Octocorallia. Page 206 in R.C. Moore, ed., Treatise on Invertebrate Paleontology Part F.
Coelenterata. The University of Kansas Press and the Geological Society of America. University of
Kansas Press, Lawrence, Kansas, U.S.A. 498 pp.
BAYER, F.M. 1961. The Shallow-water Octocorallia of the West Indian Region. A Manual for Marine Biolo-
gists. Marinus Nijhoff, The Hague, Netherlands. 373 pp. + 27 plates.
BAYER, F.M. 1981. Key to the genera of Octocorallia exclusive of Pennatulacea (Coelenterata, Anthozoa),
with diagnoses of new taxa. Proceedings of the Biological Society of Washington 94(3):902–947.
BREEDY, O., AND H.M. GUZMAN. 2014. A new species of alcyonacean octocoral from the Peruvian zoogeo-
graphic region. Journal of the Marine Biological Association of the United Kingdom 2014:1–6.
BREEDY, O., S.D. CAIRNS, AND V. HÄUSSERMANN. 2015. A new alcyonacean octocoral (Cnidaria, Anthozoa,
Octocorallia) from Chilean fjords. Zootaxa 3919(2):327–334.
CORDEIRO, R., L. VAN OFWEGEN, AND G. WILLIAMS. 2018b. World List of Octocorallia. Swiftia Duchassaing
& Michelotti, 1864. Accessed through: World Register of Marine Species at: http://www.marinespecies.
org/aphia.php?p=taxdetails&id=125314 on 2018-11-01.
CORDEIRO, R., L. VAN OFWEGEN, AND G. WILLIAMS. 2018c. World List of Octocorallia. Chromoplexaura
Williams, 2013. Accessed through: World Register of Marine Species at: http://www.marinespecies.org/
aphia.php?p=taxdetails&id=724230 on 2019-01-03.
DEICHMANN, E. 1936. The Alcyonaria of the western part of the Atlantic Ocean. Memoirs of the Museum of
Comparative Zoology at Harvard College 53:1-317.
DUCHASSAING, P., AND J. MICHELOTTI. 1864. Supplément au mèmoire sur les coralliaires des Antilles.
Mémoires de l’Academie des Sciences de Turin, Series 2(23):97–206.
EVERETT, M.V., AND L.K. PARK. 2018. From genomes to populations to communities: using genomic tech-
niques to study deep-sea coral diversity. 15th Deep-Sea Biology Symposium, Abstract 159:64.
FABRICIUS, K., AND P. ALDERSLADE. 2001. Soft Corals and Sea Fans – A Comprehensive Guide to the Tropi-
cal Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea. Australian Insti-
tute of Marine Science, Townsville, Australia. 264 pp.
GOLDBERG, W.M. 2001. The sclerites and geographic distribution of the gorgonian Swiftia exserta (Coelen-
terata: Octocorallia: Holaxonia). Bulletin of the Biological Society of Washington 10:100–109.
HARDEN, D.G. 1979. Intuitive and numerical classification of east Pacific Gorgonacea (Octocorallia). PhD
thesis, Illinois State University, USA. Unpublished [page number unkown].
KÜKENTHAL, W. 1913. Über die Alcyonarienfauna Californiens und ihre tiergeographischen Beziehungen.
Zoologische Jahrbücher (Systematik) 35(2):219–270.
KÜKENTHAL, W. 1924. Gorgonaria. Das Tierreich 47. Walter de Gruyter & Company, Berlin & Leipzig,
Germany. 478 pp.
NUTTING, C.C. 1909. Alcyonaria of the Californian coast. Proceedings of the U.S. National Museum
35(1658):681–727.
VERRILL, A.E. 1883. Report on the Anthozoa, and some additional species dredged by the “Blake” in 1877–
1879, and by the U.S. Fish Steamer “Fish Hawk” in 1880–82. Bulletin of the Comparative Museum of
Zoology, Harvard 11:1–72.
VERRILL, A.E..1928. Hawaiian shallow-water Anthozoa. Bernice P. Bishop Museum Bulletin 49:1–30.
WILLIAMS, G.C. 2013. New taxa and revisionary systematics of alcyonacean octocorals from the Pacific coast
of North America (Cnidaria, Anthozoa). ZooKeys 283:15–42.
WILLIAMS, G.C., AND O. BREEDY. 2016. A new species of whip-like gorgonian coral in the genus Swiftia from
148 PROCEEDINGS OF THE CALIFORNIA ACADEMY OF SCIENCES
Series 4, Volume 65, No. 6
the Gulf of the Farallones in Central California, with a key to eastern Pacific species in California
(Cnidaria, Octocorallia, Plexauridae). Proceedings of the California Academy of Sciences, ser. 4, 63(1):
1–13.
WRIGHT, E.P., AND T. STUDER. 1889. Report on the Alcyonaria collected by H.M.S. Challenger during the years
1873–1876. Report on the Scientific Results of the Voyage Challenger, During the Years 1873–1876.
Zoology 31:1–314.




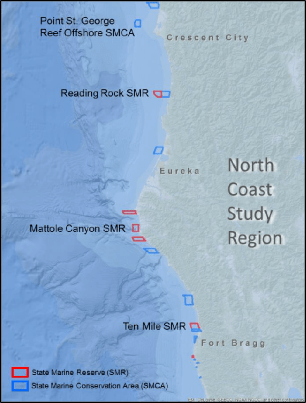
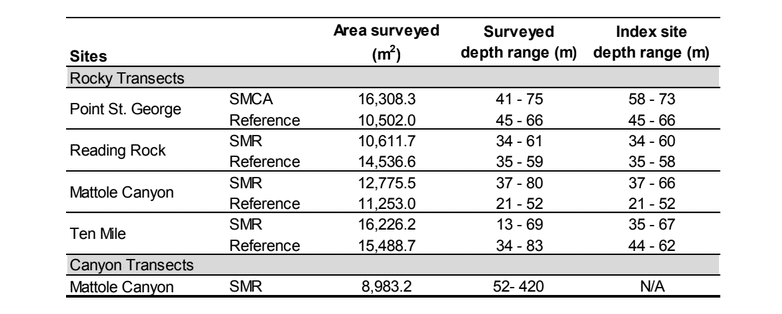
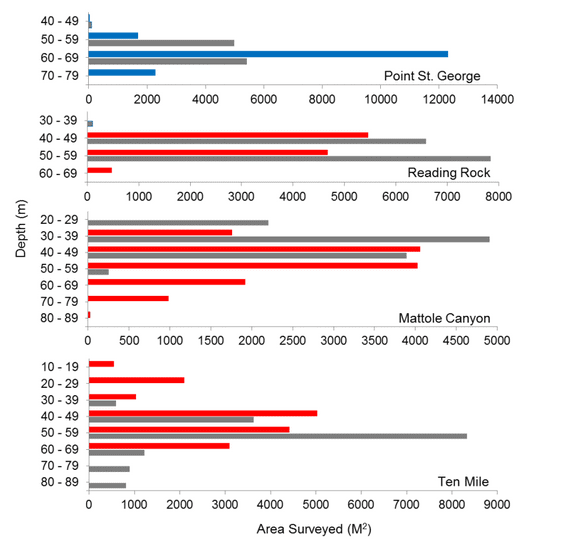
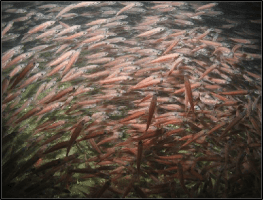 This network was implemented in four uniquely distinct coastal regions. The final region to be implemented, the North Coast Study Region (NCSR), extends from the California-Oregon border to Alder Creek, Mendocino County. The NCSR has some of the least developed coastal areas in the state due to the remote, rugged coastline and frequently unfavorable ocean conditions, which limit access to much of the coastal and offshore waters. The NCSR lies within one of only four major temperate upwelling systems in the world, making it one of the most highly productive ecosystems that supports diverse and abundant assemblages of fish and invertebrate species.
This network was implemented in four uniquely distinct coastal regions. The final region to be implemented, the North Coast Study Region (NCSR), extends from the California-Oregon border to Alder Creek, Mendocino County. The NCSR has some of the least developed coastal areas in the state due to the remote, rugged coastline and frequently unfavorable ocean conditions, which limit access to much of the coastal and offshore waters. The NCSR lies within one of only four major temperate upwelling systems in the world, making it one of the most highly productive ecosystems that supports diverse and abundant assemblages of fish and invertebrate species.  Marine Applied Research and Exploration (MARE) preformed quantitative baseline surveys within the NCSR in 2014 and 2015 with the overall goal to describe the condition of three distinctive priority ecosystem features within four MPAs and their adjacent reference study areas: a) mid-depth rock ecosystems, b) soft- bottom subtidal ecosystems, and c) deep ecosystems (including canyons).
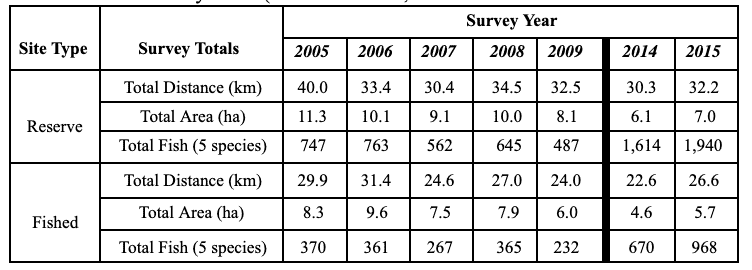
Marine Applied Research and Exploration (MARE) preformed quantitative baseline surveys within the NCSR in 2014 and 2015 with the overall goal to describe the condition of three distinctive priority ecosystem features within four MPAs and their adjacent reference study areas: a) mid-depth rock ecosystems, b) soft- bottom subtidal ecosystems, and c) deep ecosystems (including canyons).  were conducted using a remotely operated vehicle (ROV) to assess initial changes in fishes, macro-invertebrates and associated seafloor habitats. The ROV collected video and still imagery while moving along a fixed path (transect) along the sea floor. Video imagery collected was analyzed to characterize substrate, habitat types, habitat complexity (rugosity), and estimate finfish and macro-invertebrate distribution, relative abundance and density. In total, 60 ROV dives were completed surveying more than 106 km (19 ha) between 13 and 421 m deep. In addition, over 16,500 still photos were taken.
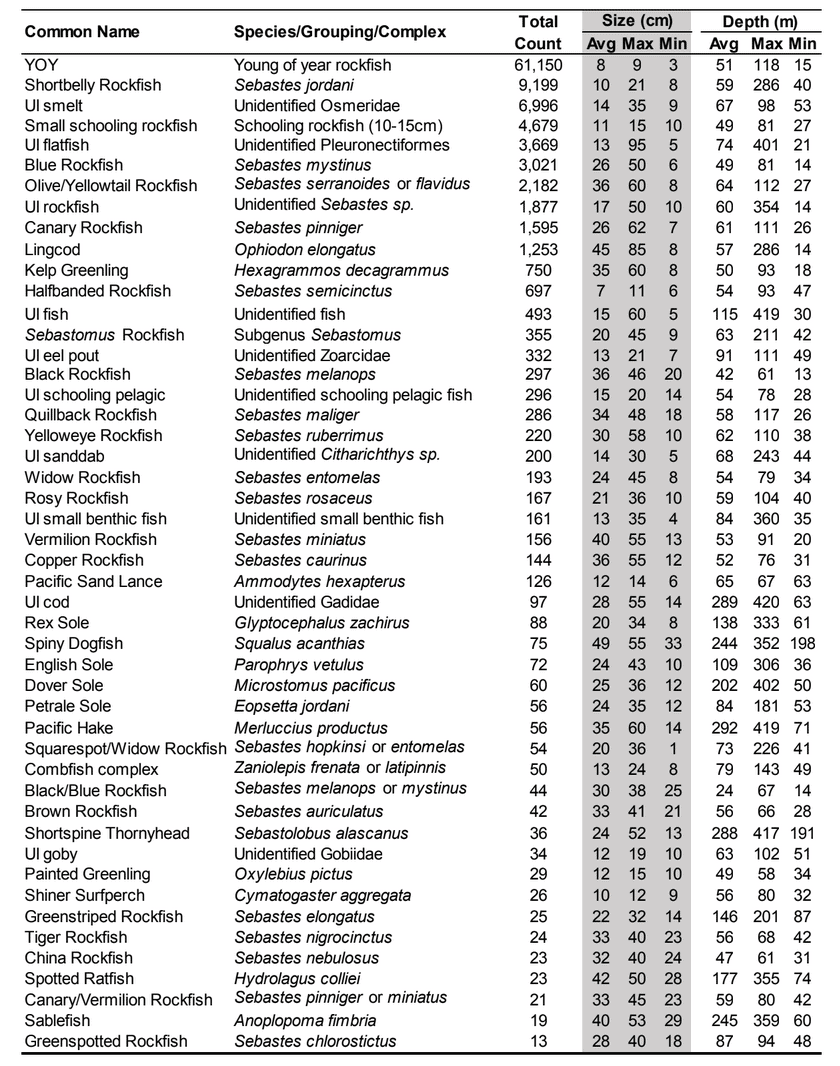
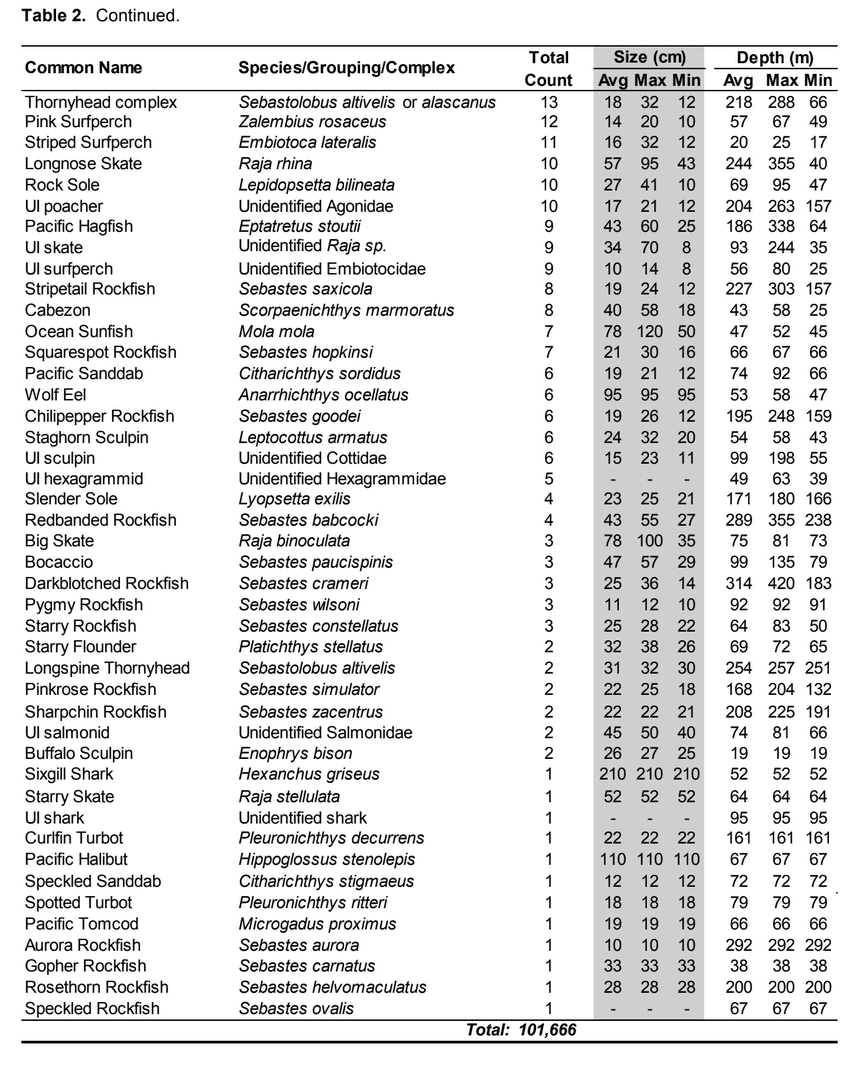
were conducted using a remotely operated vehicle (ROV) to assess initial changes in fishes, macro-invertebrates and associated seafloor habitats. The ROV collected video and still imagery while moving along a fixed path (transect) along the sea floor. Video imagery collected was analyzed to characterize substrate, habitat types, habitat complexity (rugosity), and estimate finfish and macro-invertebrate distribution, relative abundance and density. In total, 60 ROV dives were completed surveying more than 106 km (19 ha) between 13 and 421 m deep. In addition, over 16,500 still photos were taken.  Of the 101,666 fish observed over both years, over 85% were rockfish. Young of year rockfish (YOY) were the most numerous rockfish grouping observed; with over 61,000 recorded individuals, accounting for 60% of all of the fish observations. Larger rockfish represented only 14% of the rockfish identified, with four species/groupings accounting for 80% of the observations: Blue, Olive/Yellowtail, unidentified and Canary Rockfishes. Non-rockfish species represented 15% of the fish identifications, with two groupings accounting for 71% of the total observations: unidentified smelt and
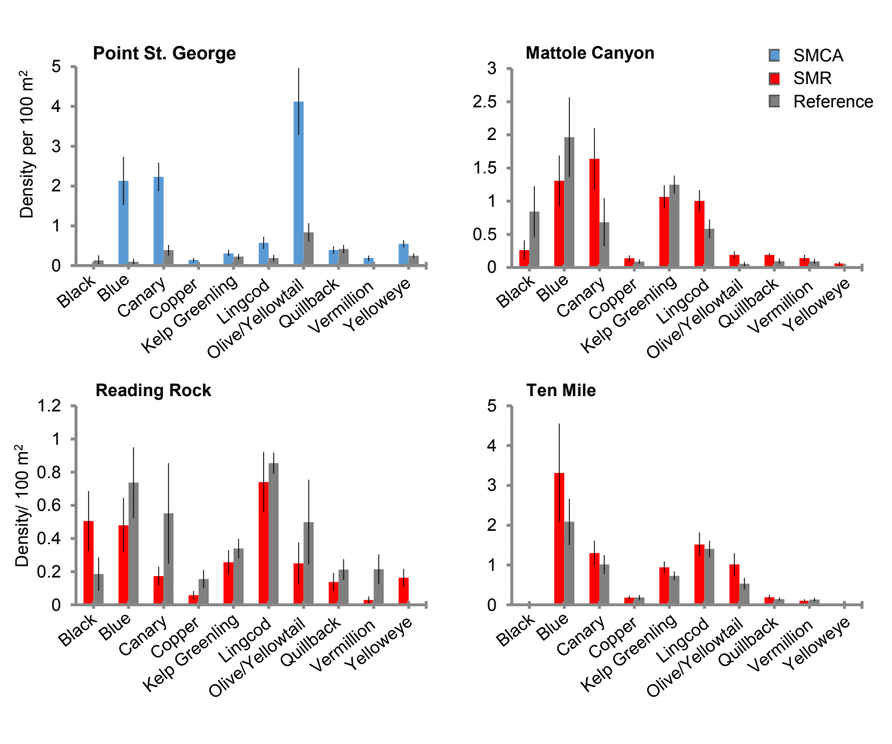
Of the 101,666 fish observed over both years, over 85% were rockfish. Young of year rockfish (YOY) were the most numerous rockfish grouping observed; with over 61,000 recorded individuals, accounting for 60% of all of the fish observations. Larger rockfish represented only 14% of the rockfish identified, with four species/groupings accounting for 80% of the observations: Blue, Olive/Yellowtail, unidentified and Canary Rockfishes. Non-rockfish species represented 15% of the fish identifications, with two groupings accounting for 71% of the total observations: unidentified smelt and  flatfish. Overall, fish species composition and density was similar between all MPA and reference area pairs within rocky reef and soft bottom habitats. One exception was documented at Point St. George Reef Offshore SMCA within rocky reef habitats, where rockfish densities were significantly lower in the reference area than the MPA (Figure E1). In addition, Point St. George Reef Offshore SMCA had the highest density of Yelloweye Rockfish, over two times more than any other study area (Figure E1).
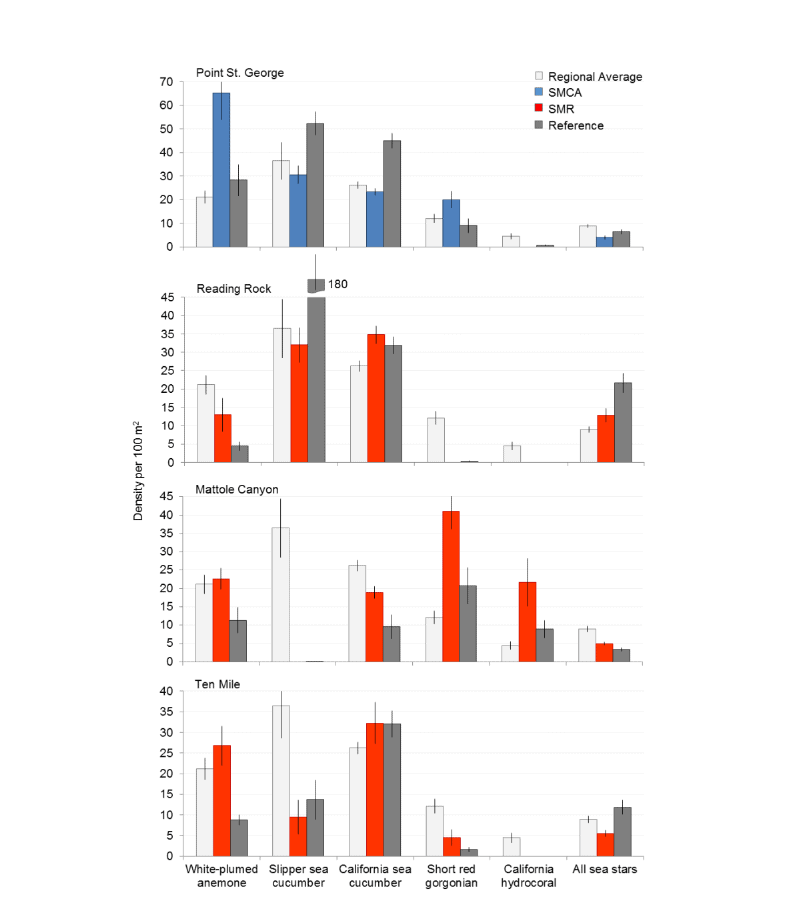
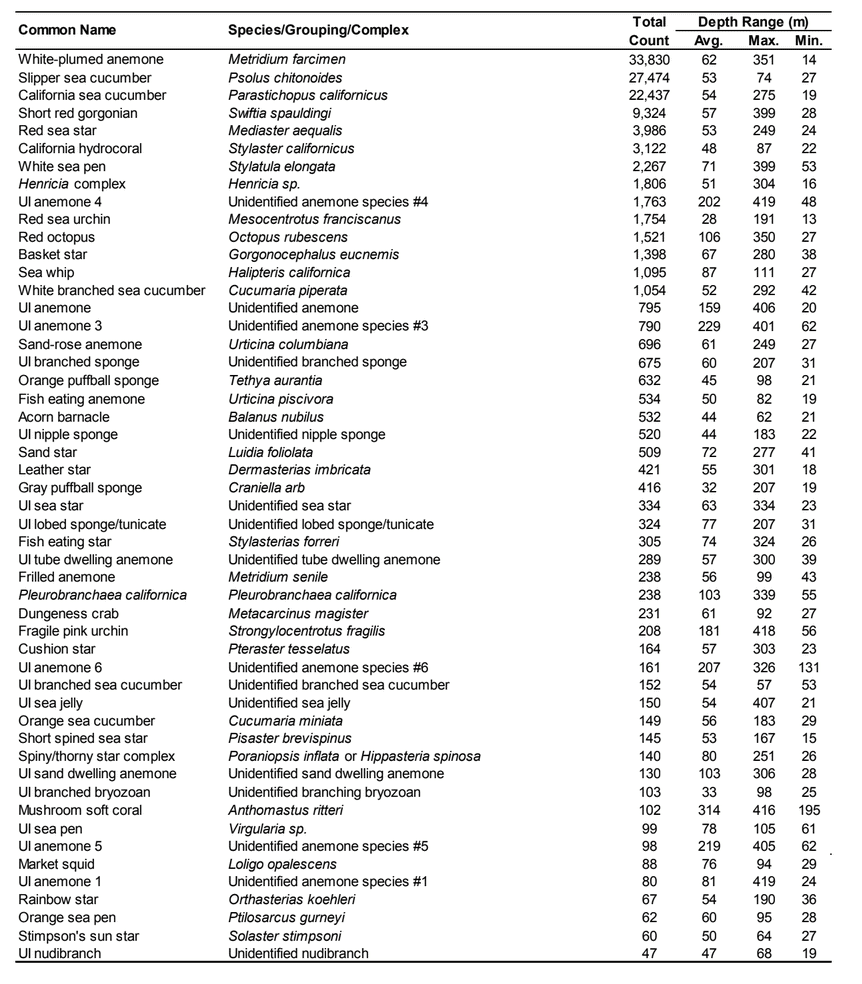
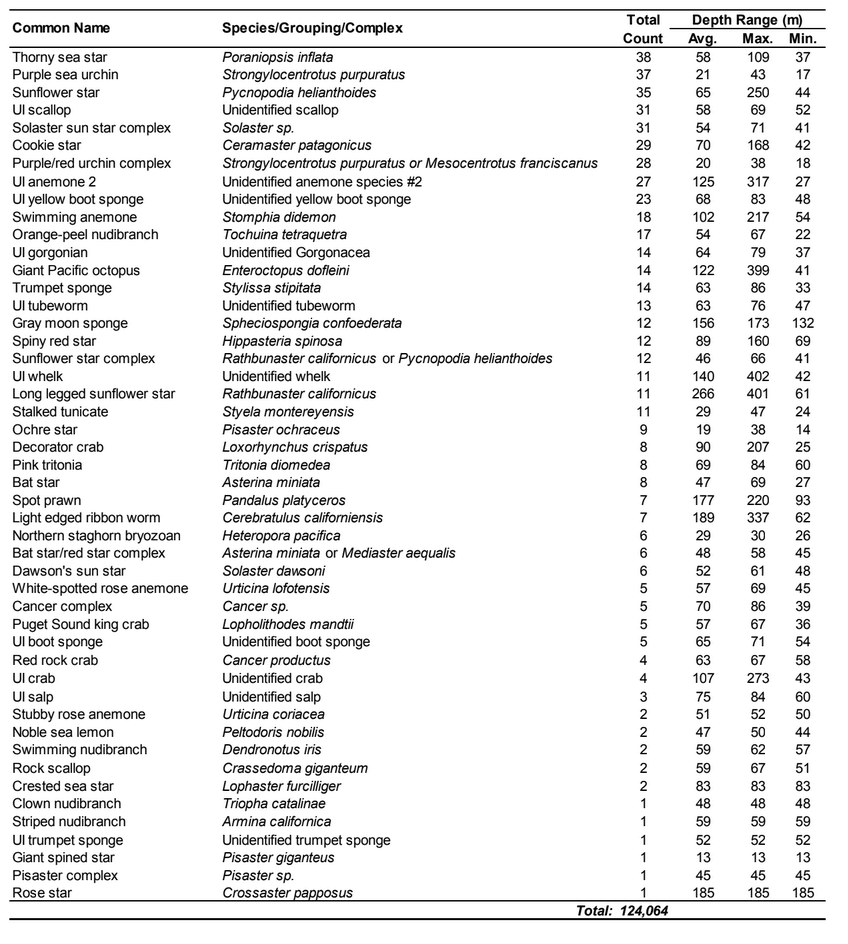
flatfish. Overall, fish species composition and density was similar between all MPA and reference area pairs within rocky reef and soft bottom habitats. One exception was documented at Point St. George Reef Offshore SMCA within rocky reef habitats, where rockfish densities were significantly lower in the reference area than the MPA (Figure E1). In addition, Point St. George Reef Offshore SMCA had the highest density of Yelloweye Rockfish, over two times more than any other study area (Figure E1).  Of the 124,064 individual invertebrates enumerated, seven species/groupings represented the majority of the observations (approximately 89%) within the NCSR: white- plumed anemones, slipper and California sea cucumbers, short red gorgonians, California hydrocoral, sea stars, sea whips and sea pens. Overall, invertebrate species composition and density was similar between all MPA and reference pairs within rocky reef habitats (Figure E2). However, within soft-bottom habitats species composition and abundance varied greatly across study locations.
Of the 124,064 individual invertebrates enumerated, seven species/groupings represented the majority of the observations (approximately 89%) within the NCSR: white- plumed anemones, slipper and California sea cucumbers, short red gorgonians, California hydrocoral, sea stars, sea whips and sea pens. Overall, invertebrate species composition and density was similar between all MPA and reference pairs within rocky reef habitats (Figure E2). However, within soft-bottom habitats species composition and abundance varied greatly across study locations. 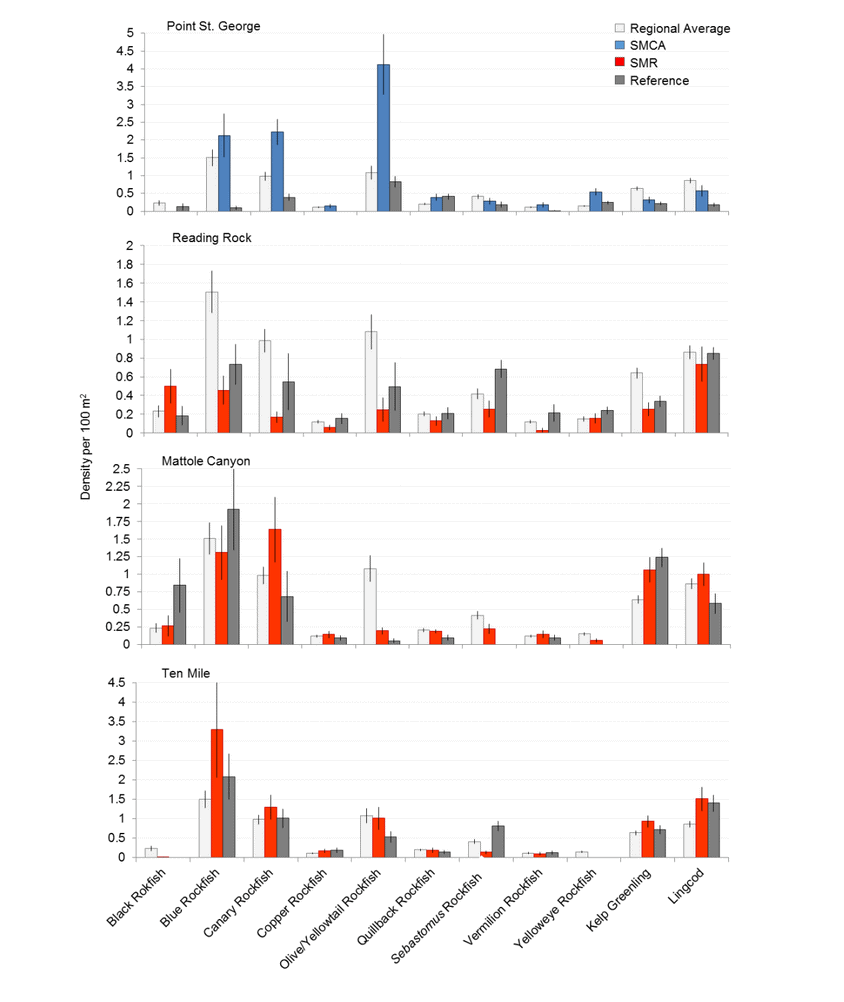

 vehicle (ROV) that is owned and operated by MARE. The ROV configuration and sampling protocols for this project were based on those developed by the project’s principal investigators in partnership with the California Department of Fish and Wildlife. The baseline assessment protocols have been used to survey MPAs of the northern Channel Islands as well as North central coast, Central coast, and South coast MPA study regions. To date, over 1,800 km of seafloor have been surveyed statewide using similar survey design and data collection protocols.
vehicle (ROV) that is owned and operated by MARE. The ROV configuration and sampling protocols for this project were based on those developed by the project’s principal investigators in partnership with the California Department of Fish and Wildlife. The baseline assessment protocols have been used to survey MPAs of the northern Channel Islands as well as North central coast, Central coast, and South coast MPA study regions. To date, over 1,800 km of seafloor have been surveyed statewide using similar survey design and data collection protocols. 
 benthic surveys of select North coast MPAs. The ROV was equipped with a three-axis autopilot including a rate gyro-damped compass and altimeter. Together, these allowed the pilot to maintain a constant heading (± 1 degree) and constant altitude (± 0.3 m) with minimal corrections. In addition, a forward speed control was used to help the pilot maintain a consistent forward velocity between 0.25 and 0.5 m/sec. A pair of Tritech® 500 kHz ranging sonars, which measure distance across a range of 0.1–10 m using a 6° conical transducer, were used as the primary method for measuring transect width for both the forward and downward facing video. Each transducer was pointed at the center of the viewing area in each camera and was used to calculate the distance to middle of screen, which was subsequently converted to width using the known properties of each cameras field of view. Readings from these sonars were averaged five times per second and recorded at a one-second interval with all other sensor data. Measurements of transect width using a ranging sonar are accurate to ± 0.1 m (Karpov et al. 2006).
benthic surveys of select North coast MPAs. The ROV was equipped with a three-axis autopilot including a rate gyro-damped compass and altimeter. Together, these allowed the pilot to maintain a constant heading (± 1 degree) and constant altitude (± 0.3 m) with minimal corrections. In addition, a forward speed control was used to help the pilot maintain a consistent forward velocity between 0.25 and 0.5 m/sec. A pair of Tritech® 500 kHz ranging sonars, which measure distance across a range of 0.1–10 m using a 6° conical transducer, were used as the primary method for measuring transect width for both the forward and downward facing video. Each transducer was pointed at the center of the viewing area in each camera and was used to calculate the distance to middle of screen, which was subsequently converted to width using the known properties of each cameras field of view. Readings from these sonars were averaged five times per second and recorded at a one-second interval with all other sensor data. Measurements of transect width using a ranging sonar are accurate to ± 0.1 m (Karpov et al. 2006).  operated by Captain Robert Pedro, was used to complete both the 2014 and 2015 surveys. Surveys were conducted between the hours of 0800 and 1700 PST to avoid the low light conditions of dawn and dusk that might affect finfish abundance measurements and underwater visibility. At each site, the ROV was piloted along pre-planned transect lines and was flown off the vessel’s port side using a “live boat” technique that employed a 317.5 kg (700 lb) clump weight. Using this method, all but 45 m of the ROV umbilical was isolated from current-induced drag by coupling it with the clump weight cable and suspending the clump weight at least 10 m off the seafloor. The 45 m tether allowed the ROV pilot sufficient maneuverability to maintain a constant speed (0.5 to 0.75 m/sec) and a straight course down the planned survey line.
operated by Captain Robert Pedro, was used to complete both the 2014 and 2015 surveys. Surveys were conducted between the hours of 0800 and 1700 PST to avoid the low light conditions of dawn and dusk that might affect finfish abundance measurements and underwater visibility. At each site, the ROV was piloted along pre-planned transect lines and was flown off the vessel’s port side using a “live boat” technique that employed a 317.5 kg (700 lb) clump weight. Using this method, all but 45 m of the ROV umbilical was isolated from current-induced drag by coupling it with the clump weight cable and suspending the clump weight at least 10 m off the seafloor. The 45 m tether allowed the ROV pilot sufficient maneuverability to maintain a constant speed (0.5 to 0.75 m/sec) and a straight course down the planned survey line. 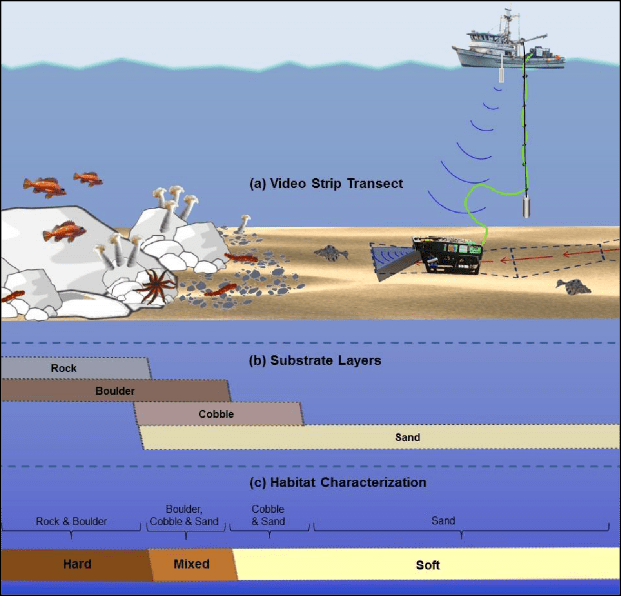
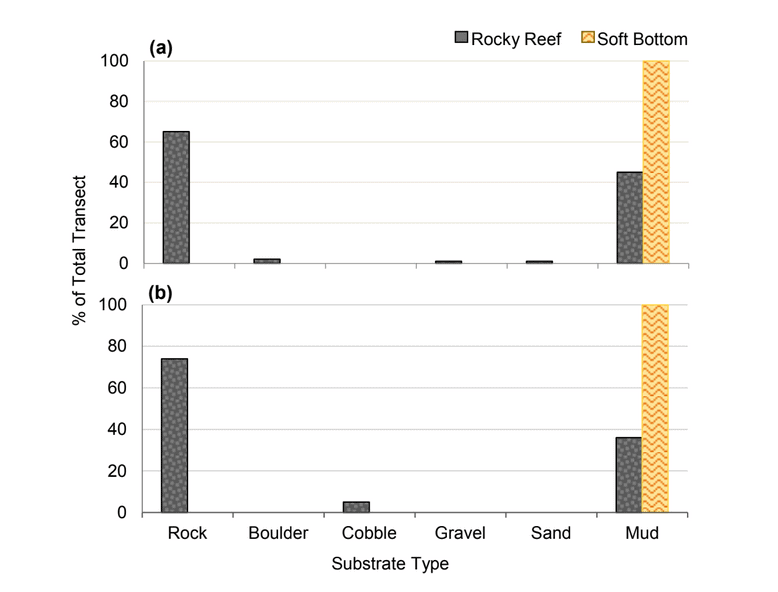
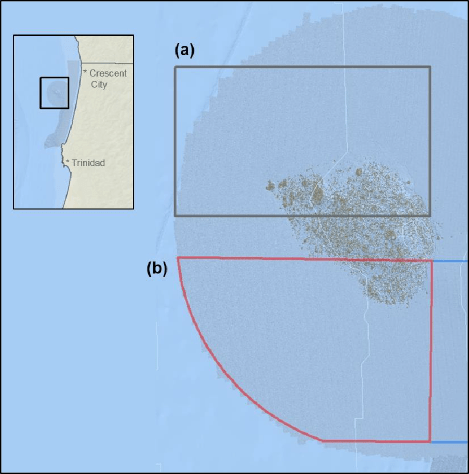
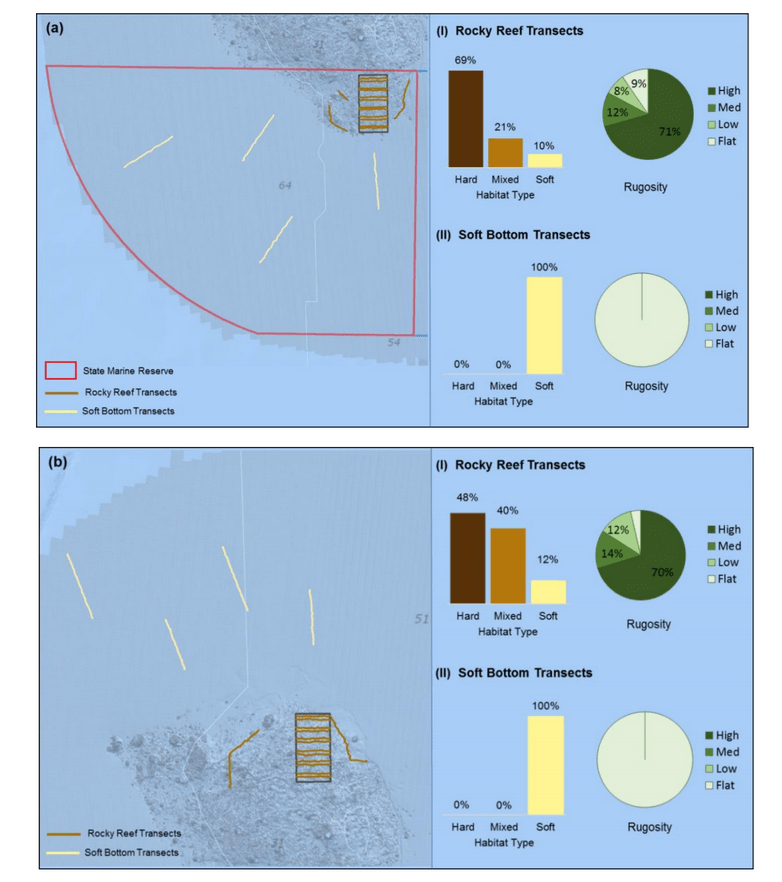
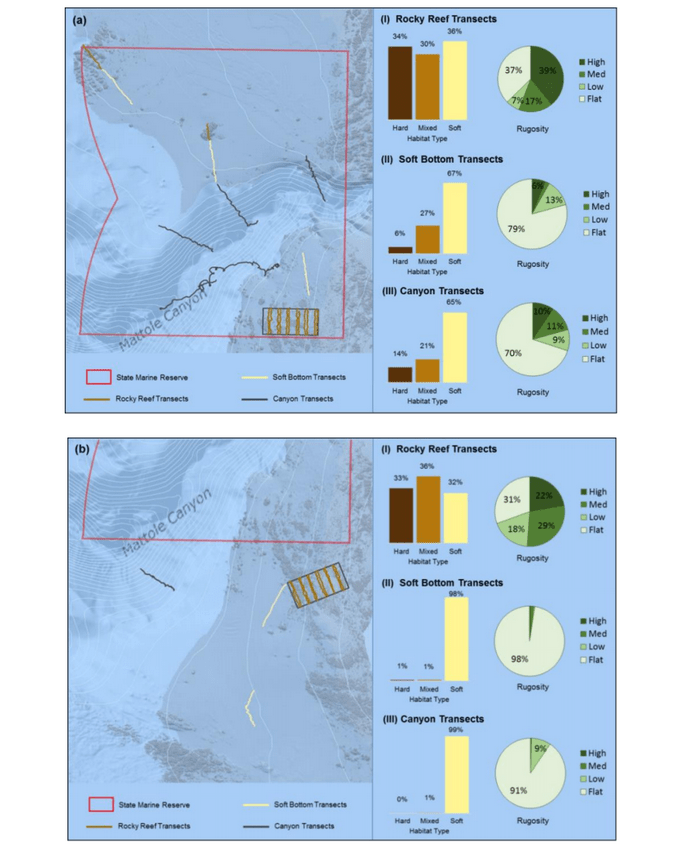
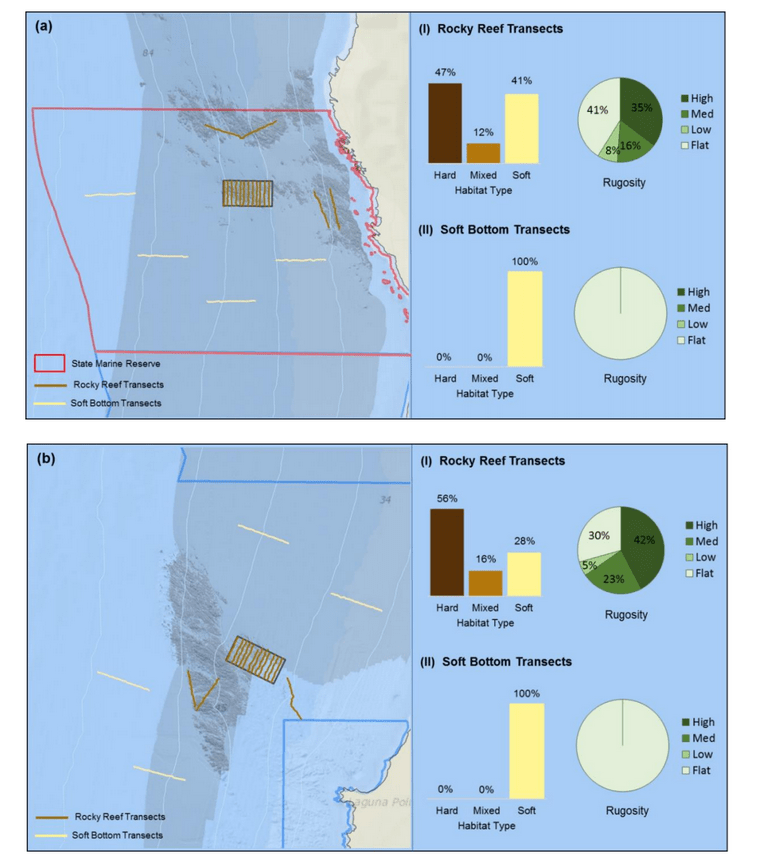
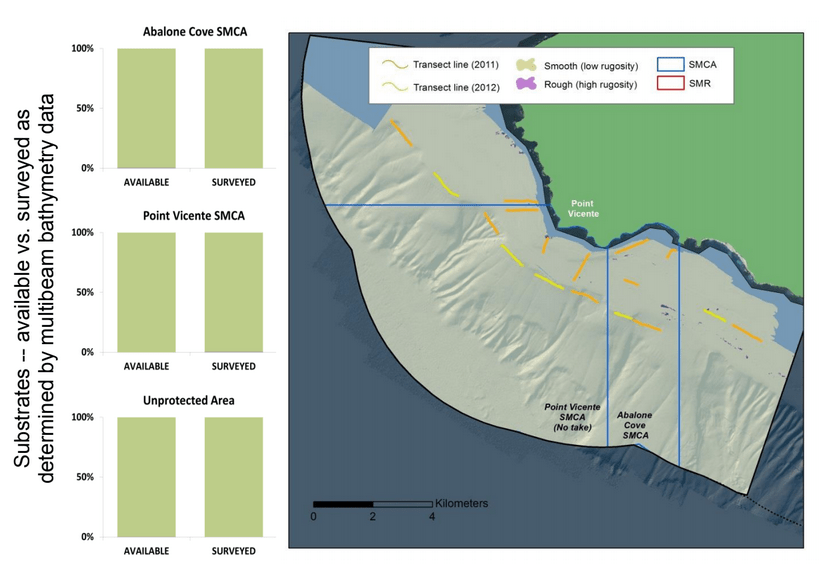
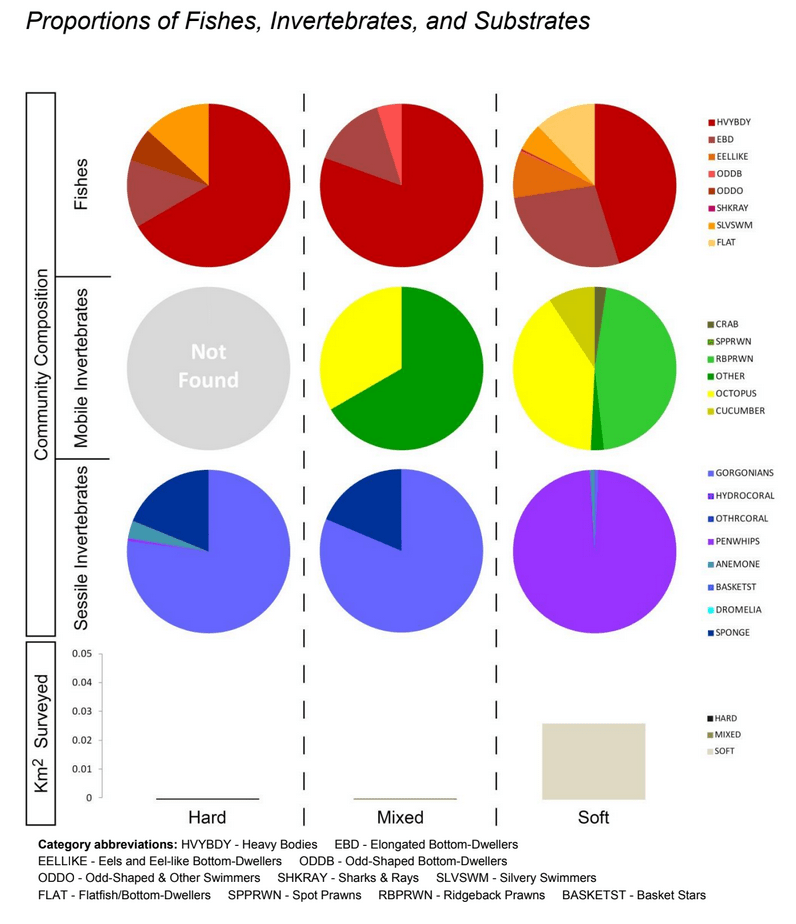
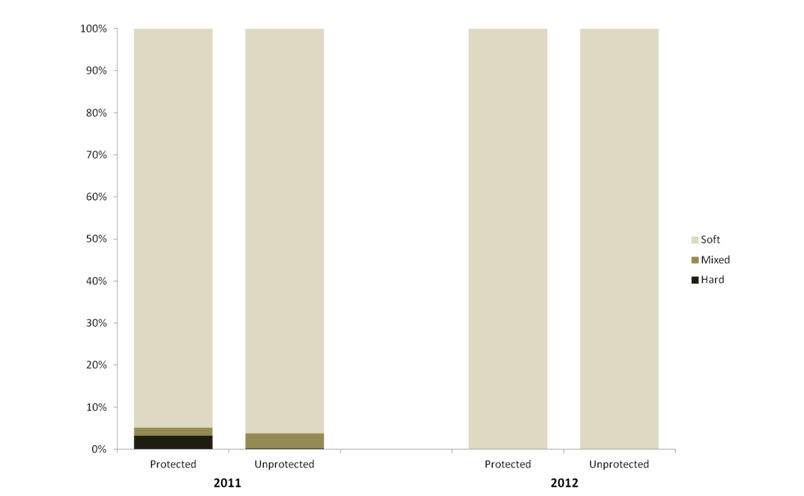
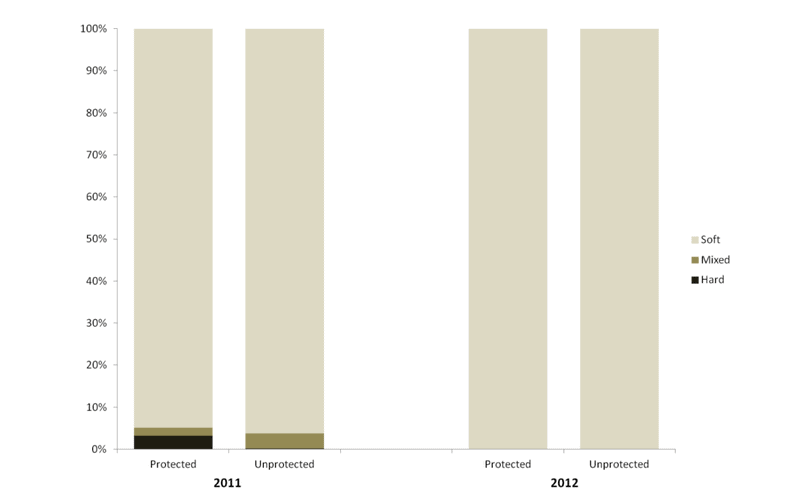
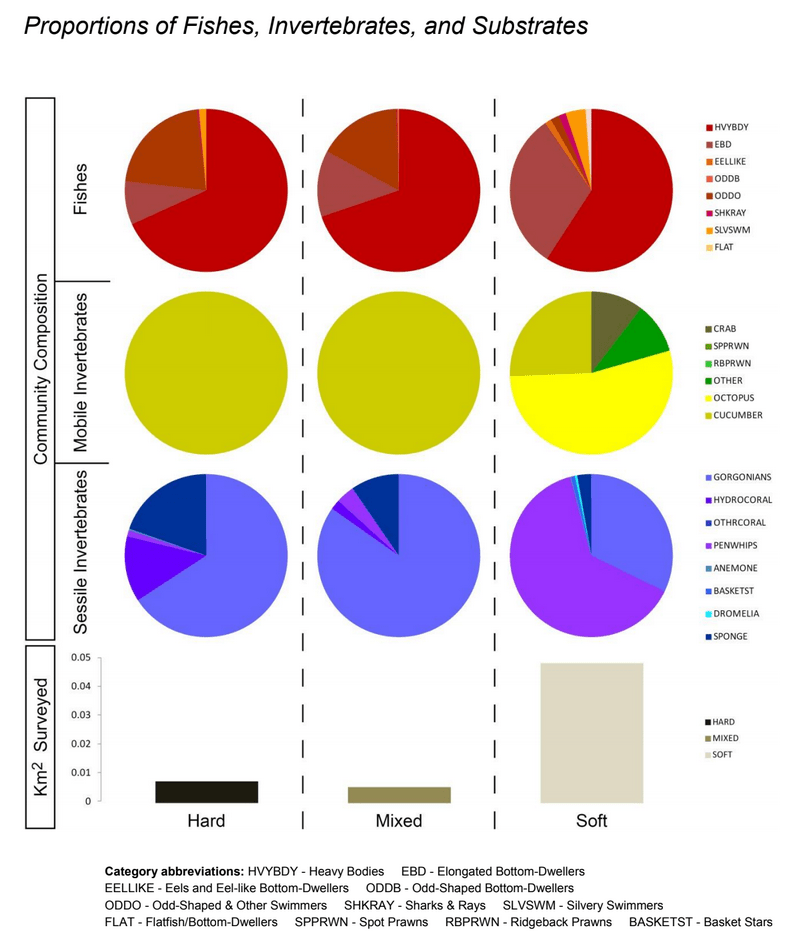
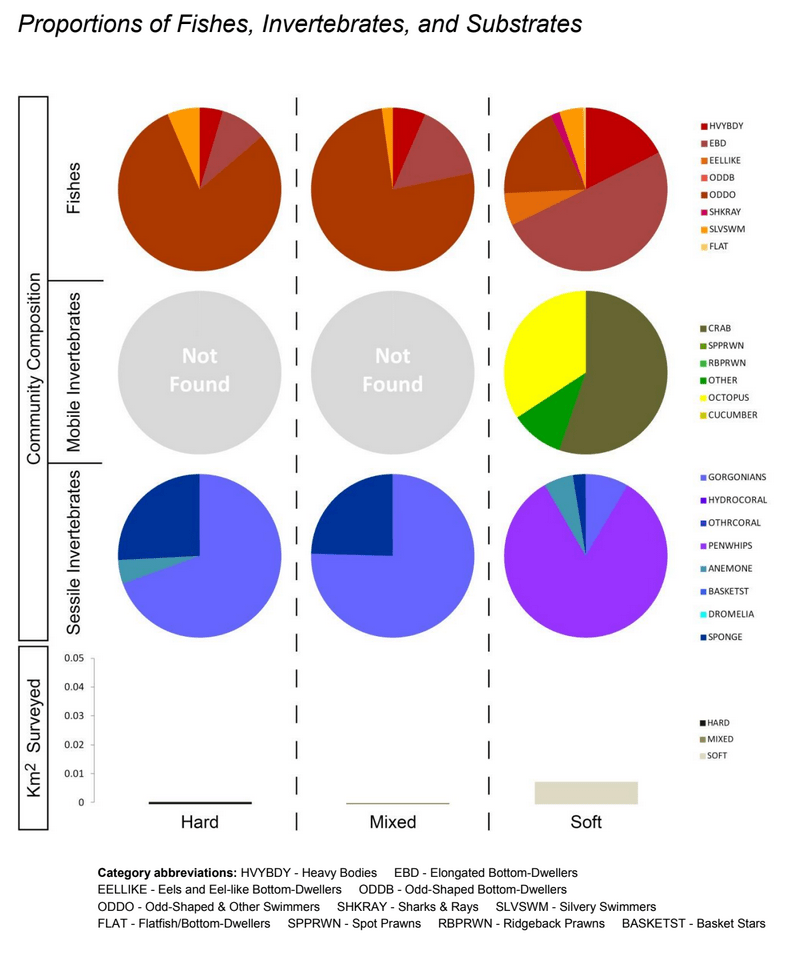
 independent habitat types: hard, soft, and mixed habitats (Figure 2). Rock and boulder were categorized as hard substrate types, while cobble, gravel, sand, and mud were all considered to be unconsolidated substrates and categorized as soft. Hard habitat was defined as any combination of the hard substrates, soft habitat as any combination of soft substrates, and mixed habitat as any combination of hard and soft substrates.
independent habitat types: hard, soft, and mixed habitats (Figure 2). Rock and boulder were categorized as hard substrate types, while cobble, gravel, sand, and mud were all considered to be unconsolidated substrates and categorized as soft. Hard habitat was defined as any combination of the hard substrates, soft habitat as any combination of soft substrates, and mixed habitat as any combination of hard and soft substrates. 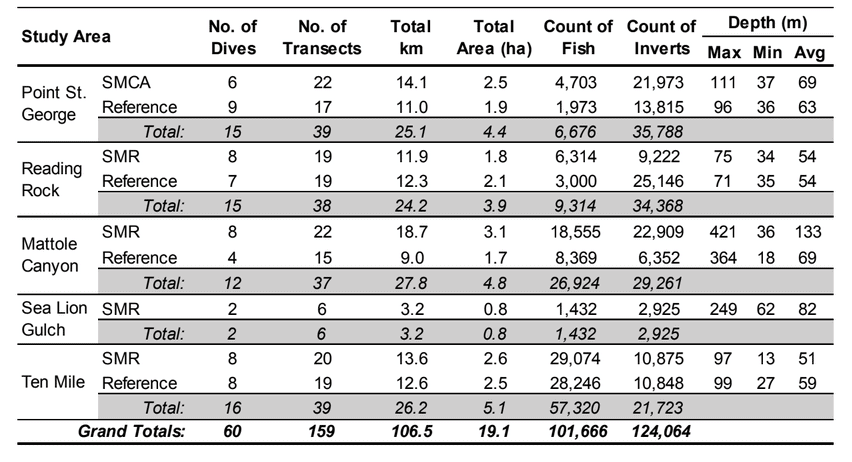
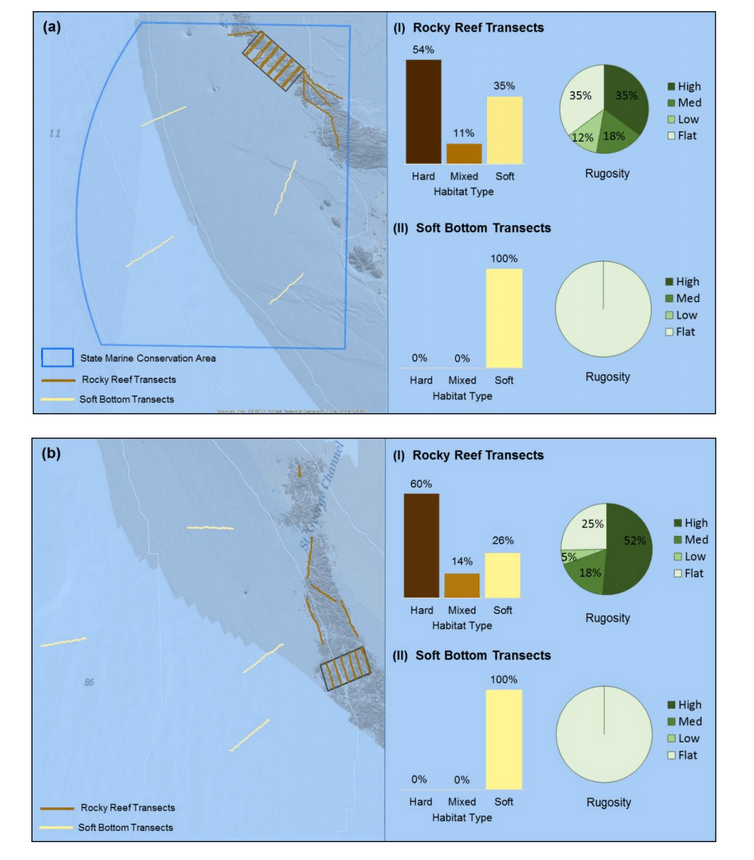
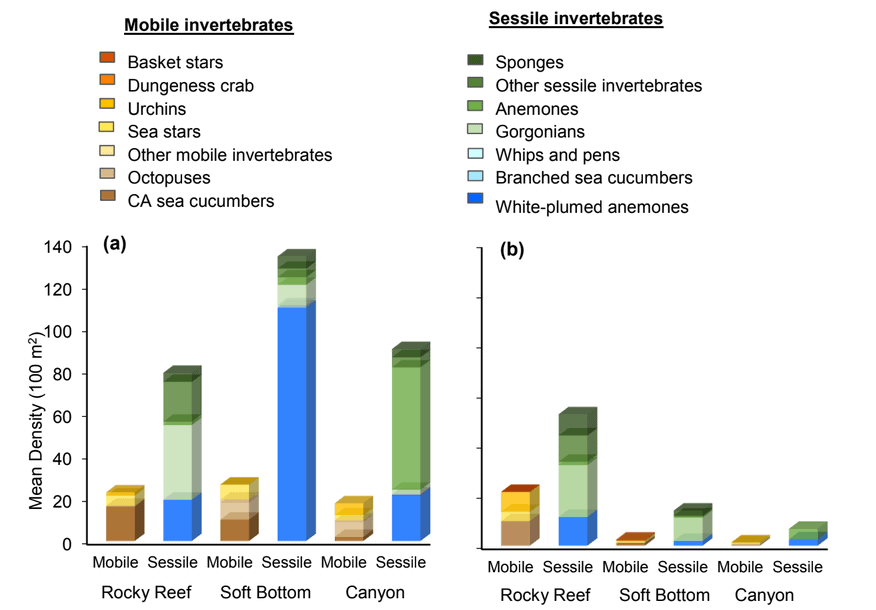
 study areas was similar (Figure 8). Five macro-invertebrate species dominated observations within the rocky reef of both the SMCA and reference area: white-plumed anemones, slipper sea cucumbers, California sea cucumbers, short red gorgonians and red sea stars. While not characteristic of both study areas, basket stars were also common within the rocky reef of the SMCA.
study areas was similar (Figure 8). Five macro-invertebrate species dominated observations within the rocky reef of both the SMCA and reference area: white-plumed anemones, slipper sea cucumbers, California sea cucumbers, short red gorgonians and red sea stars. While not characteristic of both study areas, basket stars were also common within the rocky reef of the SMCA. 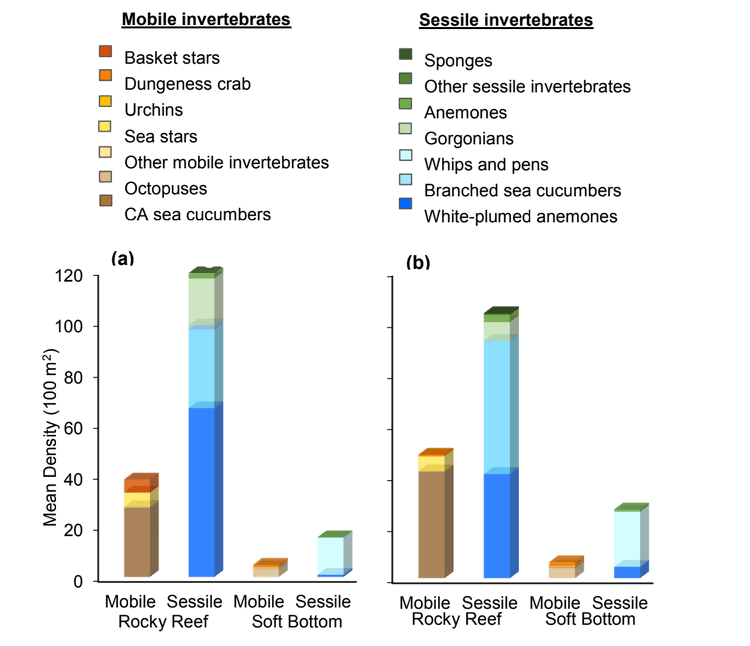
 rocky reef were composed of all 6 substrate types (Figure 10). However, the reference area contained greater amounts of boulder, cobble and gravel at the transitional portions of the rocky reef than the SMR. Transects targeting soft bottom habitats within the SMR and reference area were entirely composed of mud substrate.
rocky reef were composed of all 6 substrate types (Figure 10). However, the reference area contained greater amounts of boulder, cobble and gravel at the transitional portions of the rocky reef than the SMR. Transects targeting soft bottom habitats within the SMR and reference area were entirely composed of mud substrate. 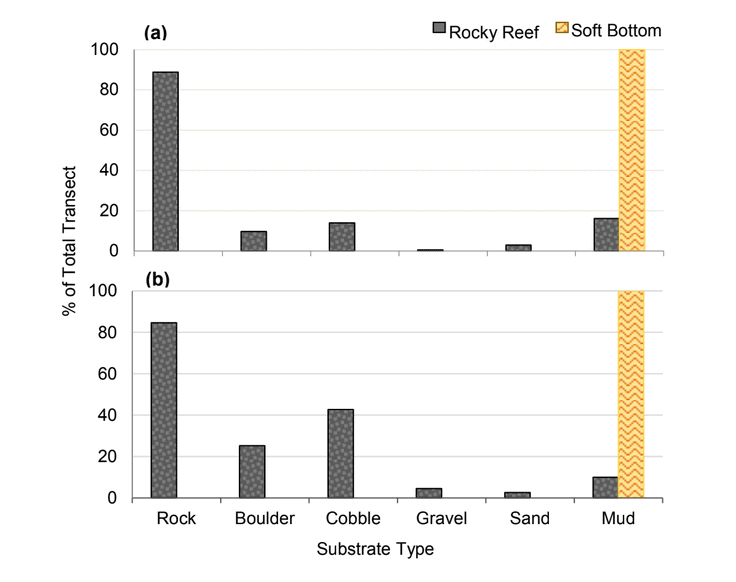
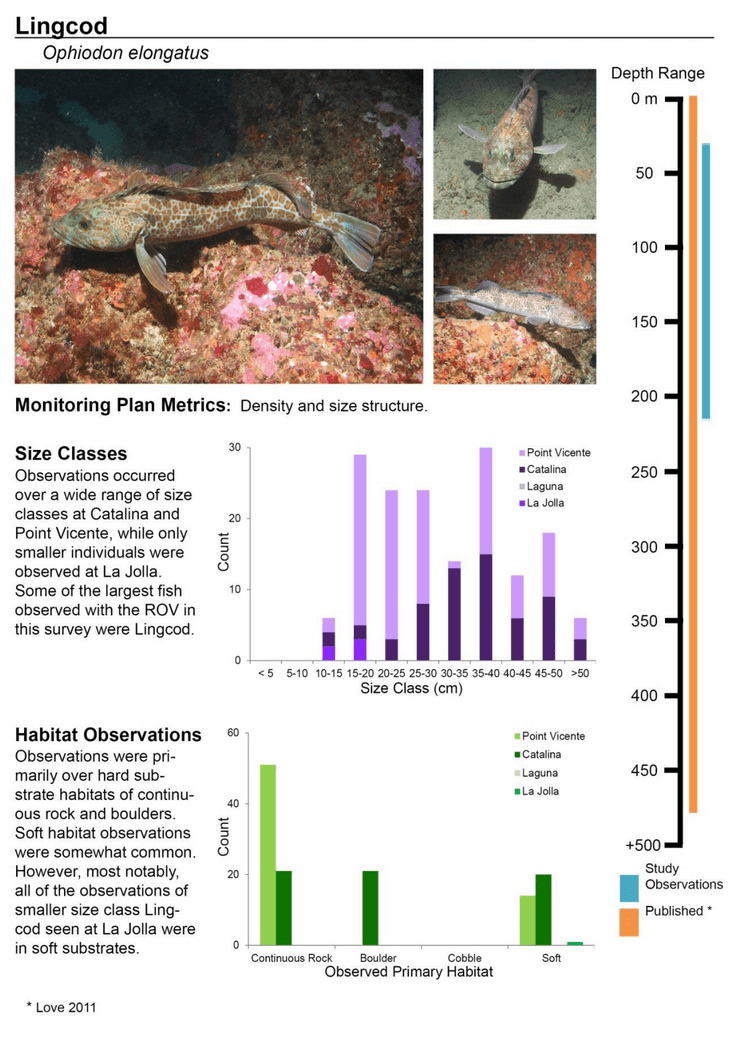
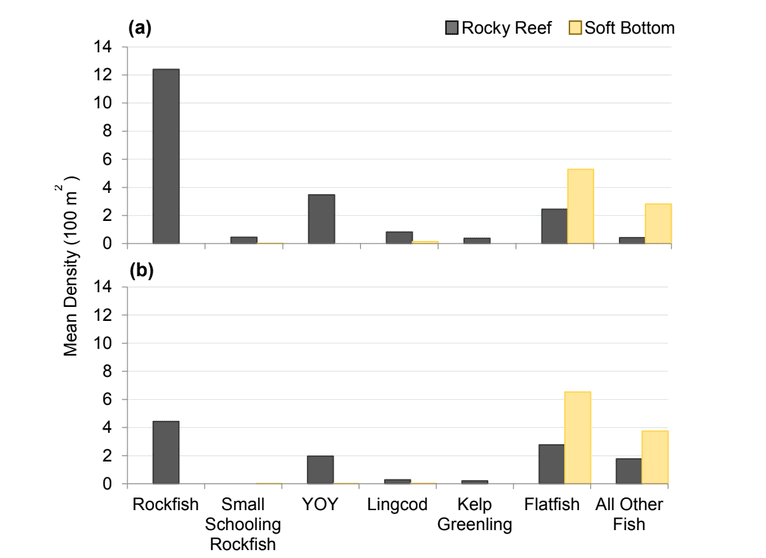
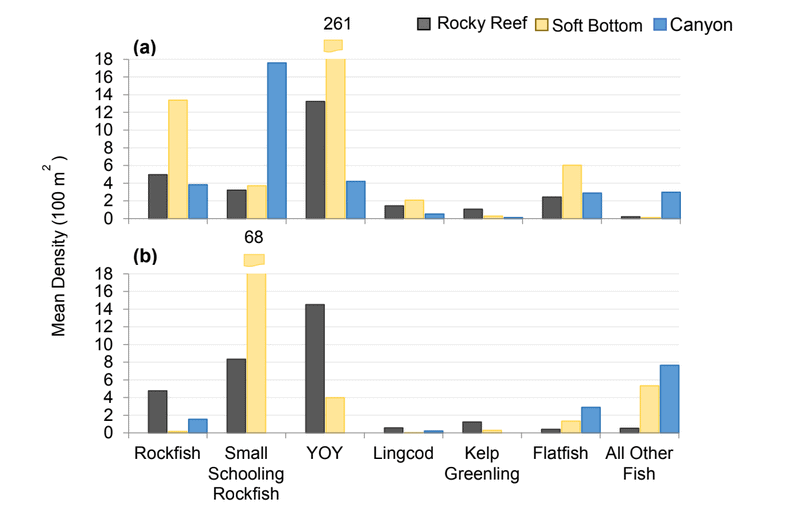
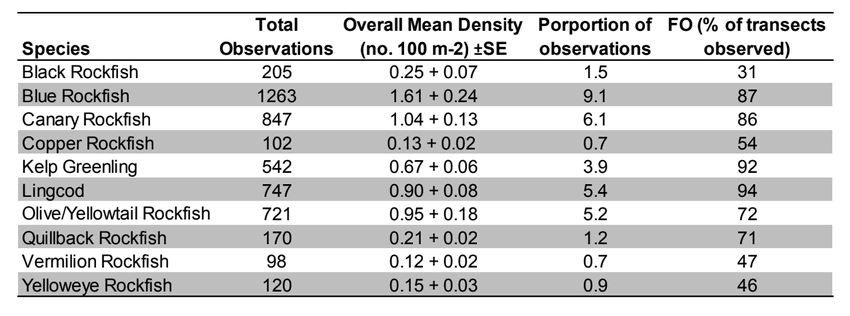
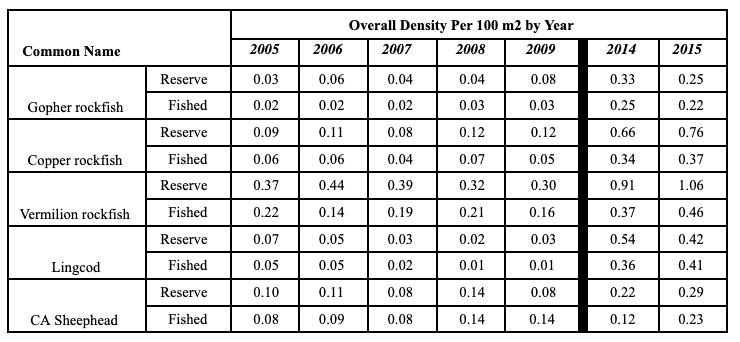
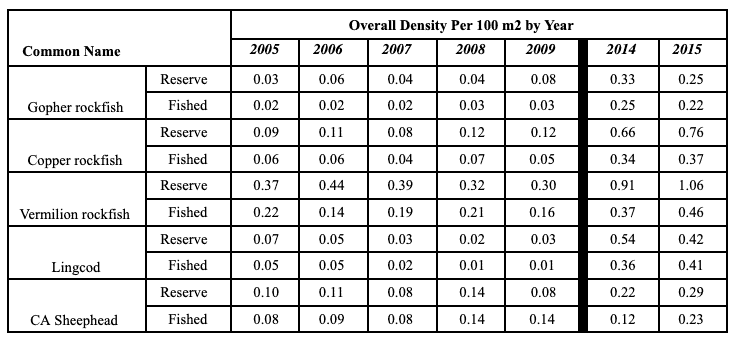
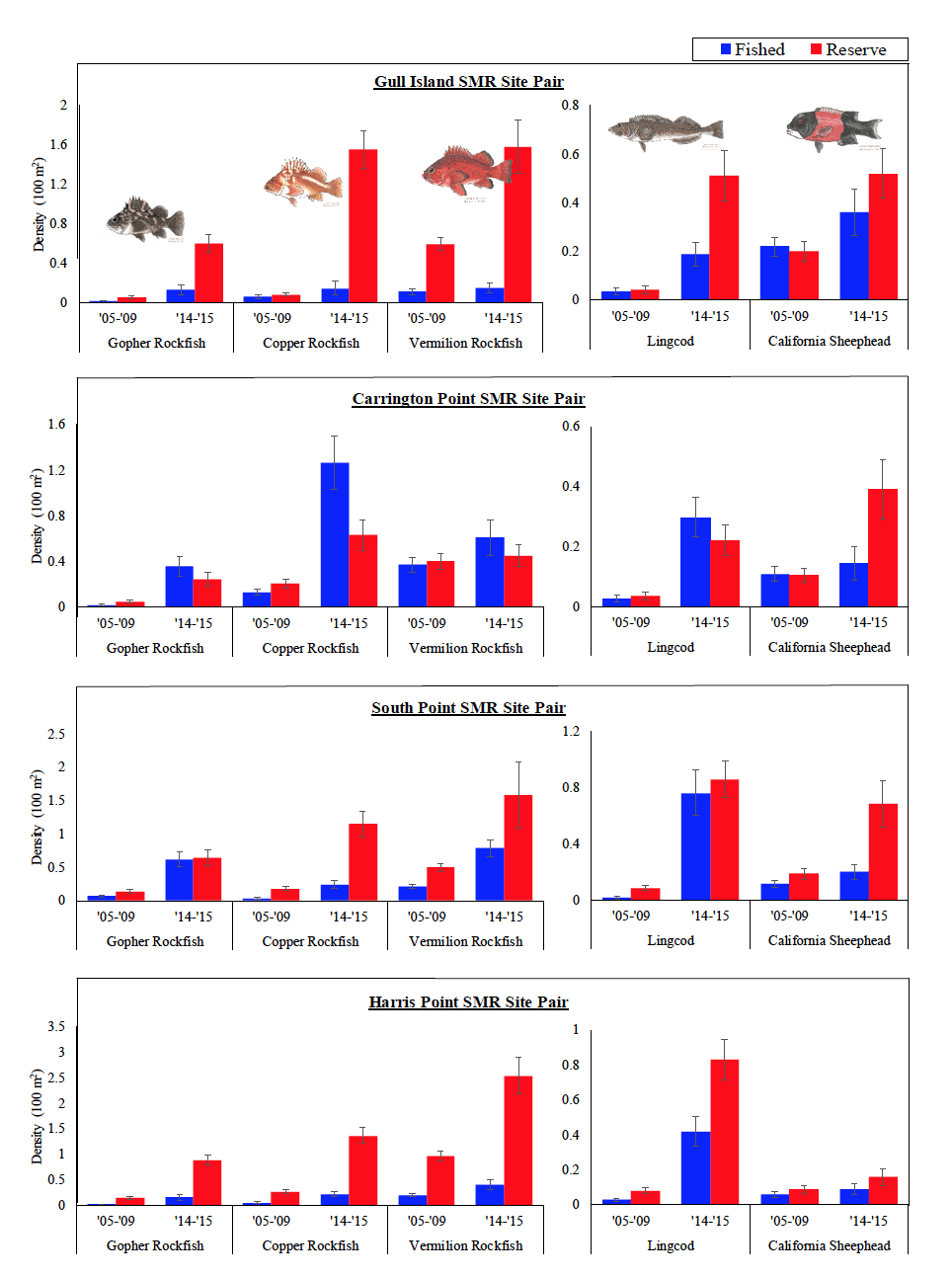
 Rockfish species, Lingcod and Kelp Greenling characterized the rocky reef, even though their densities were quite low (Figure 12). Five species/groupings of rockfish accounted for 76% of the overall rockfish subgrouping density in the SMR, and over 65% in the reference area: unidentified rockfish, Olive/Yellowtail, Black, Blue, and Canary Rockfish. The epibenthic aggregating species including Blue Rockfish and Olive/Yellowtail Rockfish were commonly observed schooling around the tops of larger rock outcroppings. Another semi-aggregating species, the Canary Rockfish, was observed schooling near the sea floor close to the transition from soft bottom to rocky reef. Canary Rockfish were also observed within the rocky reef itself, but in lower numbers. Additional benthic rockfish species, such as Yelloweye Rockfish, Quillback Rockfish, Vermilion Rockfish and Rosy Rockfish, were observed intermixed throughout the rocky reef in both study areas.
Rockfish species, Lingcod and Kelp Greenling characterized the rocky reef, even though their densities were quite low (Figure 12). Five species/groupings of rockfish accounted for 76% of the overall rockfish subgrouping density in the SMR, and over 65% in the reference area: unidentified rockfish, Olive/Yellowtail, Black, Blue, and Canary Rockfish. The epibenthic aggregating species including Blue Rockfish and Olive/Yellowtail Rockfish were commonly observed schooling around the tops of larger rock outcroppings. Another semi-aggregating species, the Canary Rockfish, was observed schooling near the sea floor close to the transition from soft bottom to rocky reef. Canary Rockfish were also observed within the rocky reef itself, but in lower numbers. Additional benthic rockfish species, such as Yelloweye Rockfish, Quillback Rockfish, Vermilion Rockfish and Rosy Rockfish, were observed intermixed throughout the rocky reef in both study areas. 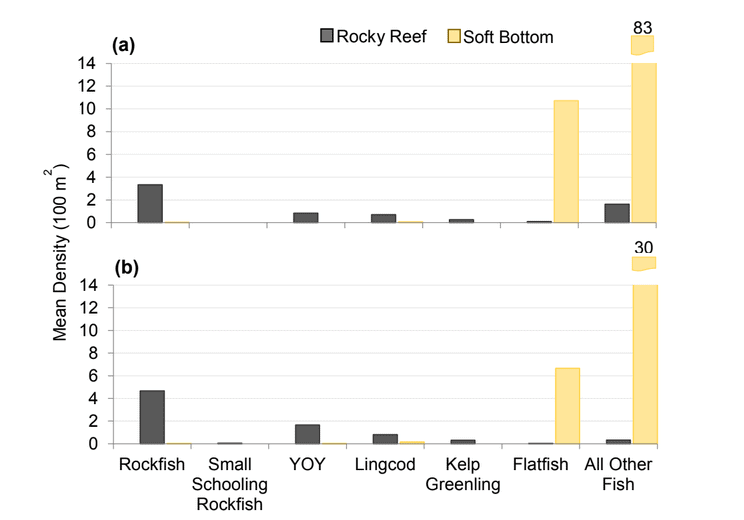
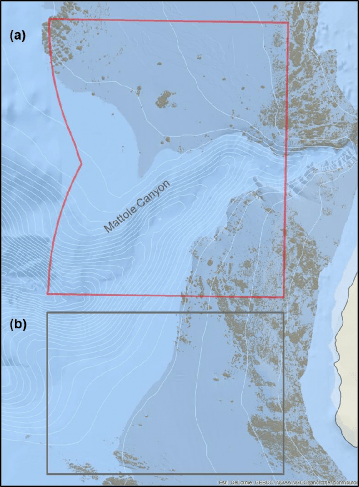
 Strong currents and large swells were common during our surveys of this location, along with strong afternoon winds and seas. These currents appeared to be characteristic of the area, as evident by the large, deep scour depressions around rock outcroppings and near the transitions between soft bottom and rocky reef. Dives targeting the canyon wall were difficult to complete due to the strong currents that swept across the shelf and accelerated down into the canyon. On the canyon floor we observed currents flushing organic materials out into deeper waters. Weather conditions changed rapidly and were not always consistent with predicted weather reports, making it a difficult location to sample. The typical work day for Mattole Canyon was short, requiring additional days beyond what was planned for this study location.
Strong currents and large swells were common during our surveys of this location, along with strong afternoon winds and seas. These currents appeared to be characteristic of the area, as evident by the large, deep scour depressions around rock outcroppings and near the transitions between soft bottom and rocky reef. Dives targeting the canyon wall were difficult to complete due to the strong currents that swept across the shelf and accelerated down into the canyon. On the canyon floor we observed currents flushing organic materials out into deeper waters. Weather conditions changed rapidly and were not always consistent with predicted weather reports, making it a difficult location to sample. The typical work day for Mattole Canyon was short, requiring additional days beyond what was planned for this study location. 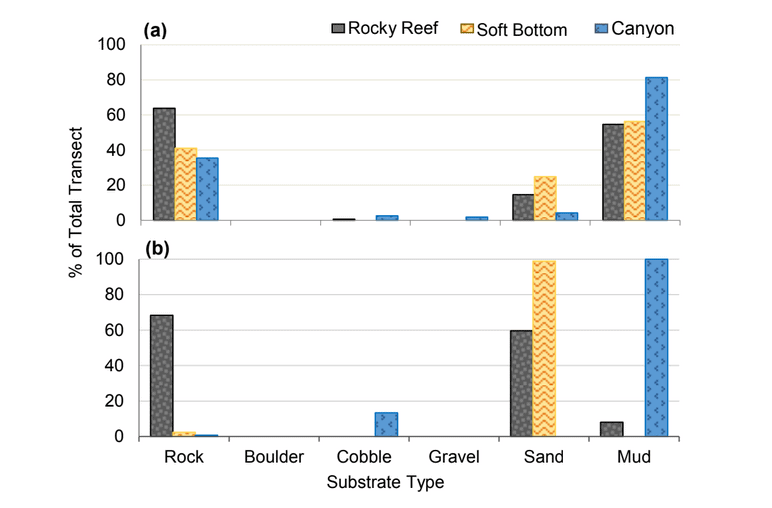

 was similar (Figure 20). At both areas, transects that targeted the rocky reef were mainly composed of rock with smaller amounts of sand and mud. Rock surfaces had less sedimentation and detritus buildup than the two northernmost study locations. These cleaner rocky surfaces hosted encrusting coralline algae in the shallower portions of the rocky reef, which was not observed at any of the other study locations. We also observed less suspended fine particulate material, which may have accounted for the better water visibility, that was typically 4 to 6 m, compared to 1 to 3 m observed at other locations such as Point St. George. Transects that targeted the soft bottom were composed exclusively of sand or mud at both study areas.
was similar (Figure 20). At both areas, transects that targeted the rocky reef were mainly composed of rock with smaller amounts of sand and mud. Rock surfaces had less sedimentation and detritus buildup than the two northernmost study locations. These cleaner rocky surfaces hosted encrusting coralline algae in the shallower portions of the rocky reef, which was not observed at any of the other study locations. We also observed less suspended fine particulate material, which may have accounted for the better water visibility, that was typically 4 to 6 m, compared to 1 to 3 m observed at other locations such as Point St. George. Transects that targeted the soft bottom were composed exclusively of sand or mud at both study areas. 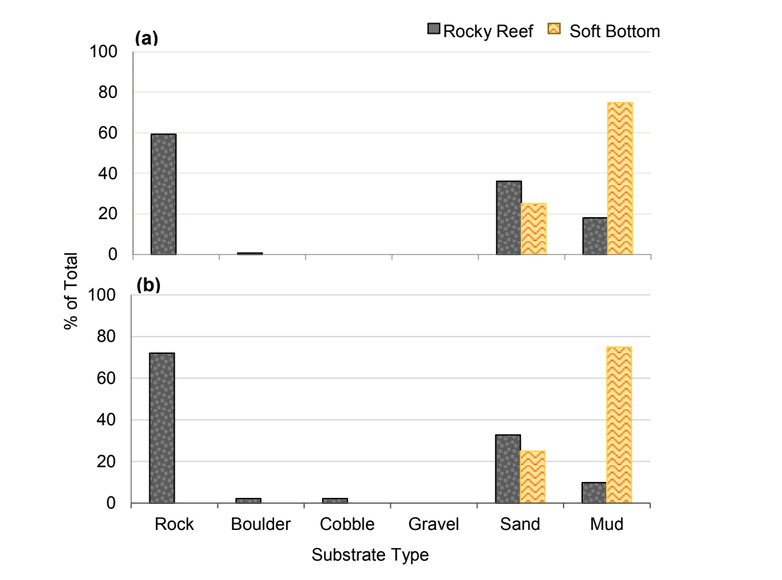
 and were mainly composed of flatfish and ‘all other fish’. The ‘all other fish’ subgroup was most notably different between the two areas. Unidentified smelt were observed in large numbers at the reference area accounting for 90% of the ‘all other fish’ density, compared to only 18% within the SMCA.
and were mainly composed of flatfish and ‘all other fish’. The ‘all other fish’ subgroup was most notably different between the two areas. Unidentified smelt were observed in large numbers at the reference area accounting for 90% of the ‘all other fish’ density, compared to only 18% within the SMCA. 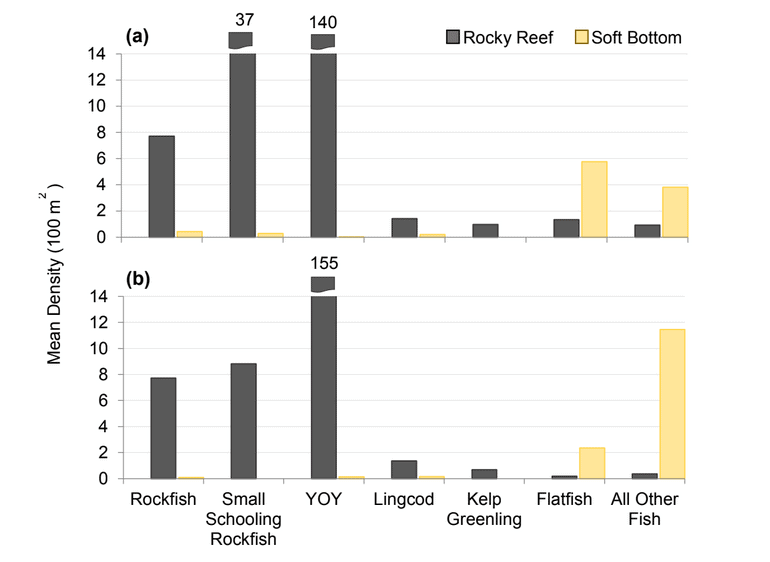
 soft bottom habitats was similar between the two study areas (Figure 23). Within the rocky reef, like the northernmost two MPAs we studied, white-plumed anemones and slipper sea cucumbers were the characteristic sessile macro-invertebrates, while California sea cucumbers were the characteristic mobile invertebrate. However, unlike the northernmost MPAs, sponges and other anemones were also abundant in the rocky reef at the Ten Mile study location.
soft bottom habitats was similar between the two study areas (Figure 23). Within the rocky reef, like the northernmost two MPAs we studied, white-plumed anemones and slipper sea cucumbers were the characteristic sessile macro-invertebrates, while California sea cucumbers were the characteristic mobile invertebrate. However, unlike the northernmost MPAs, sponges and other anemones were also abundant in the rocky reef at the Ten Mile study location. 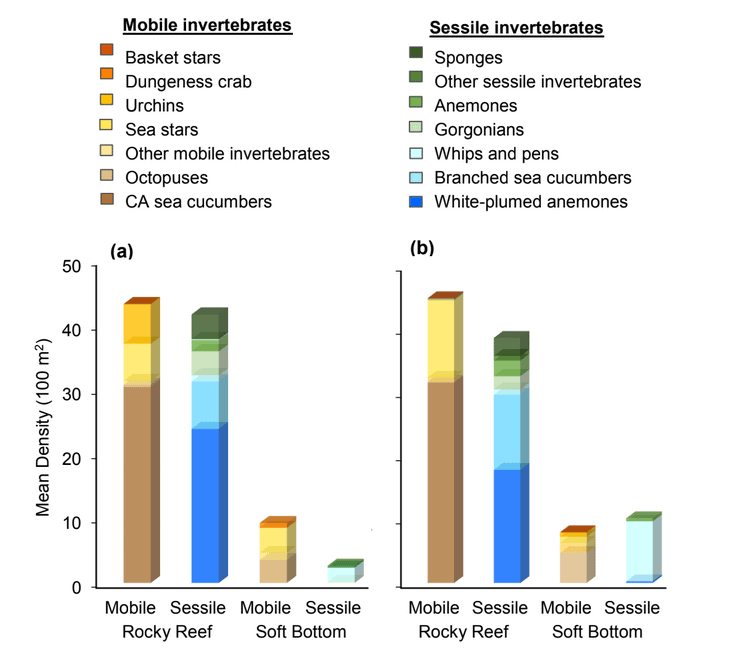







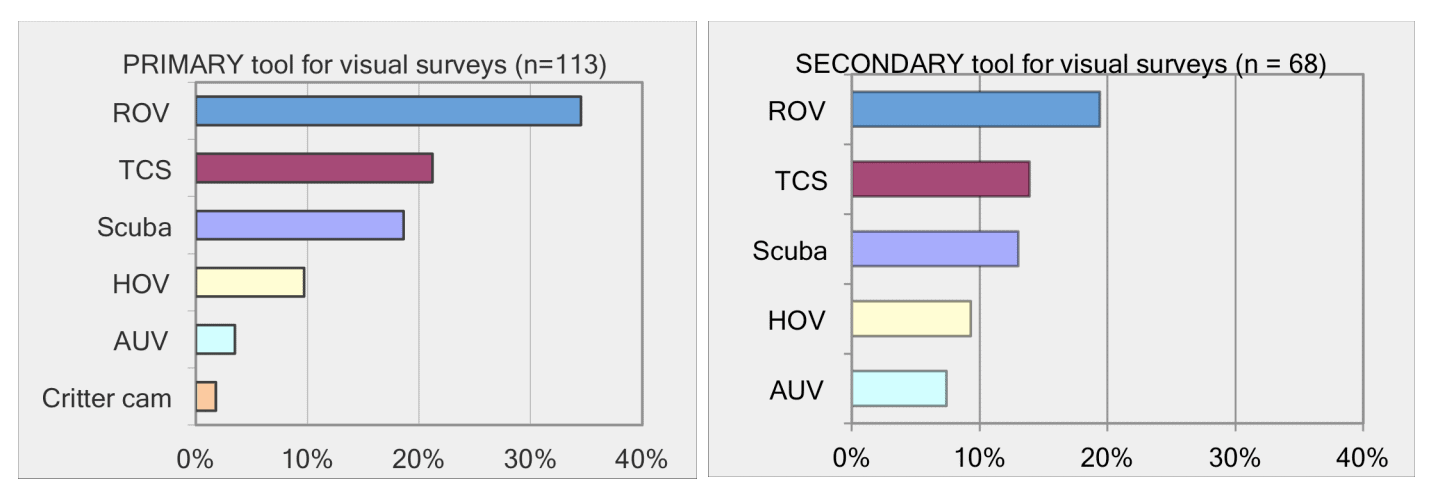
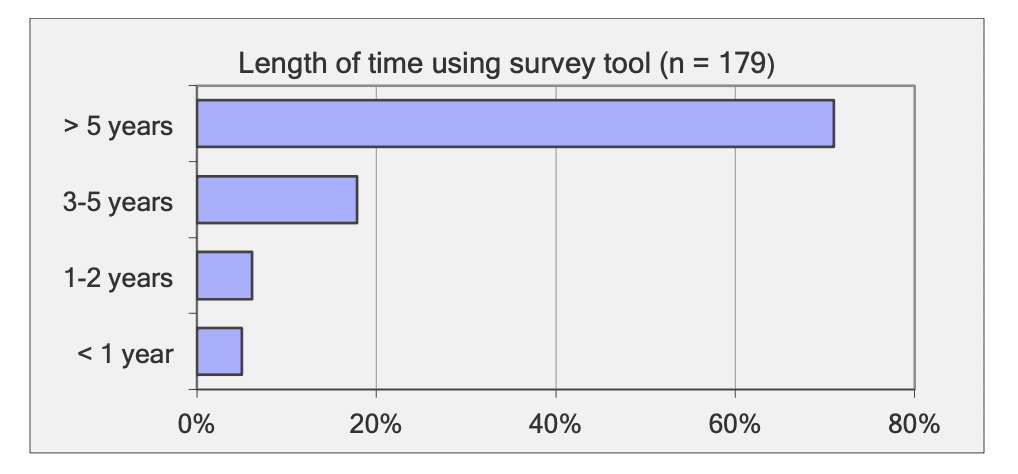
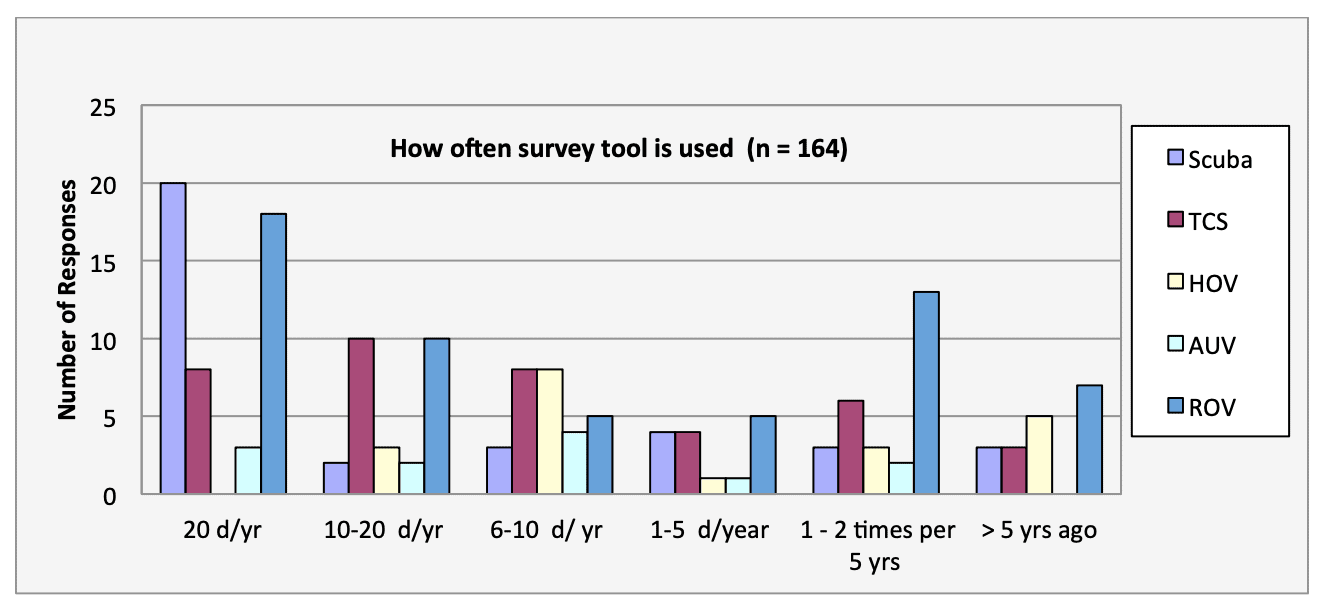
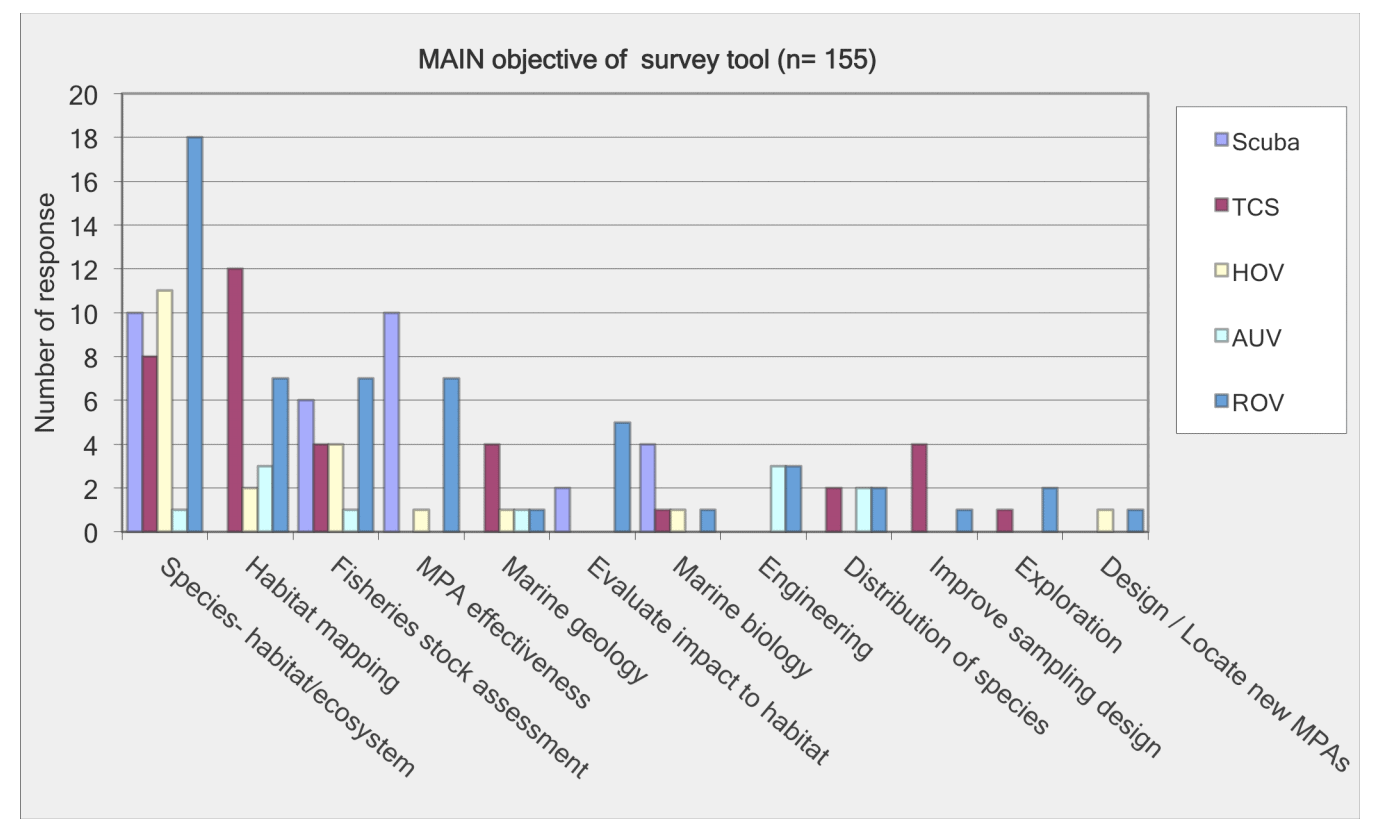
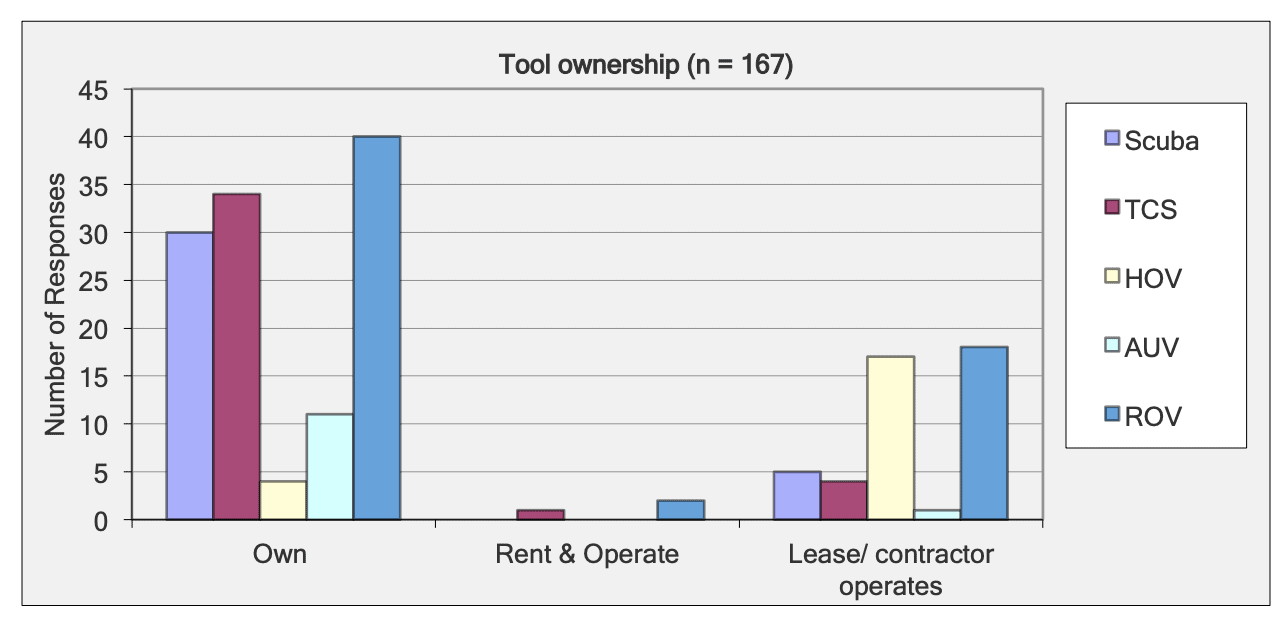
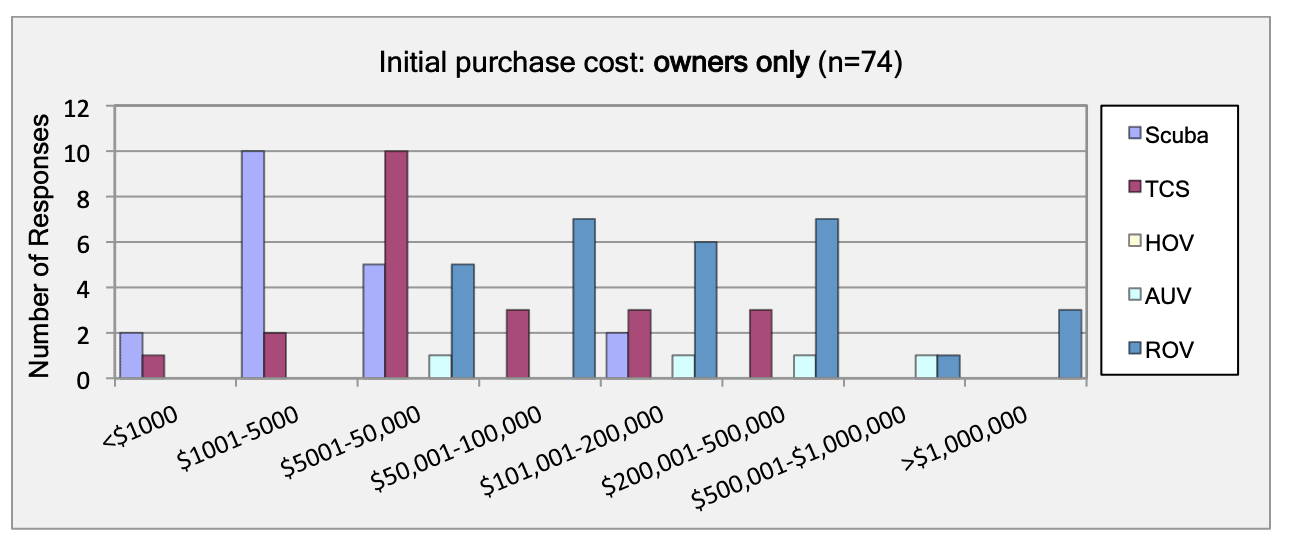
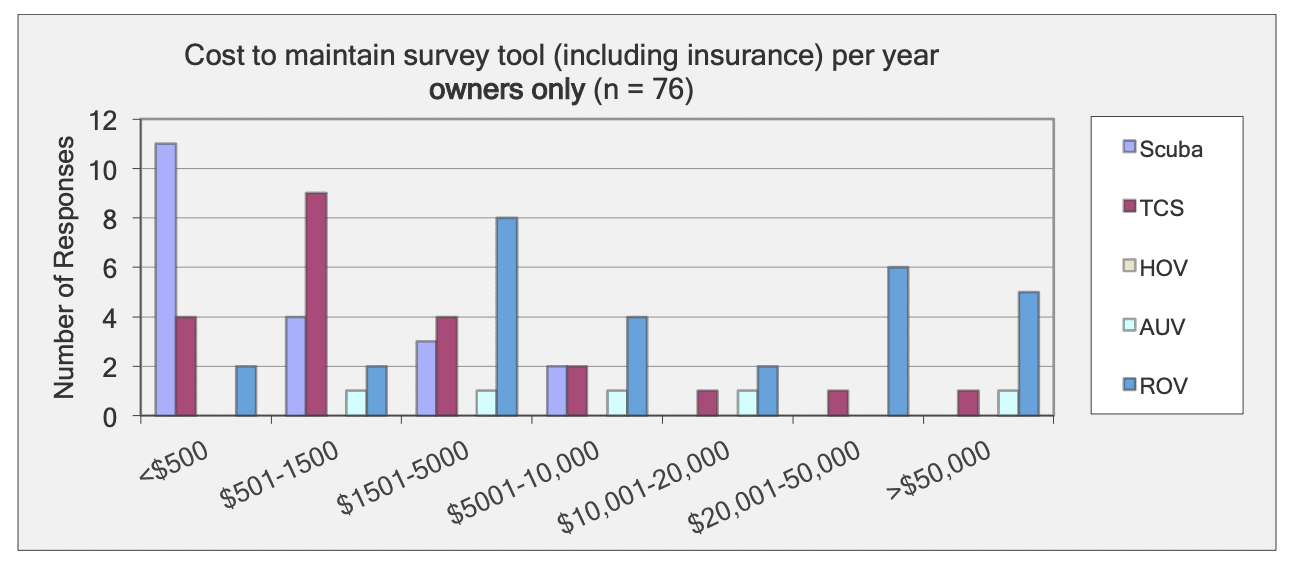
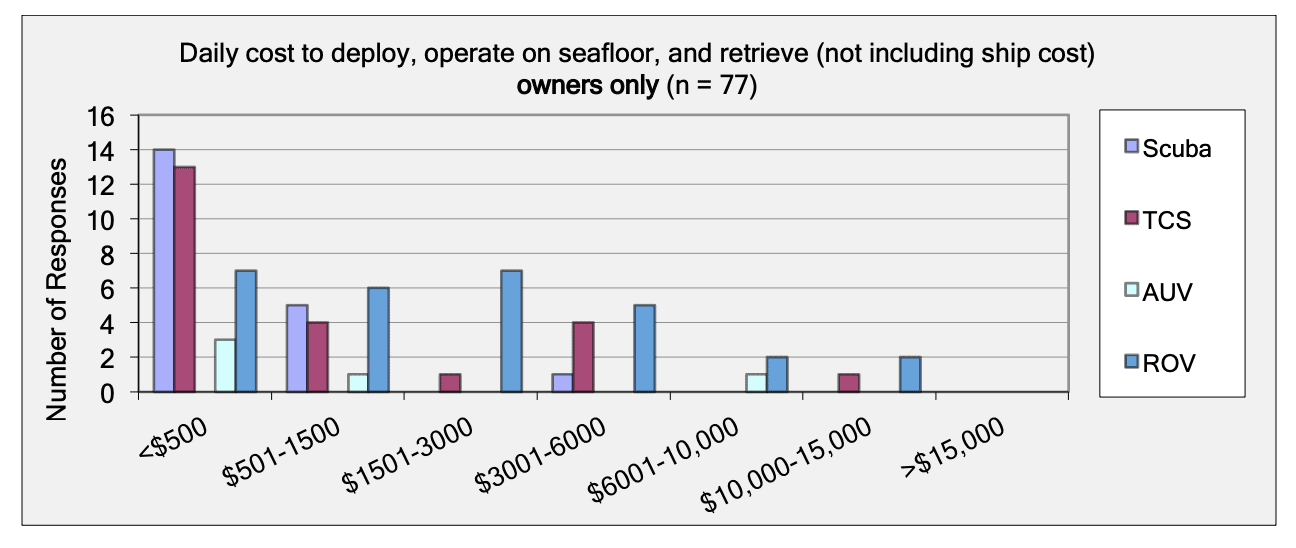
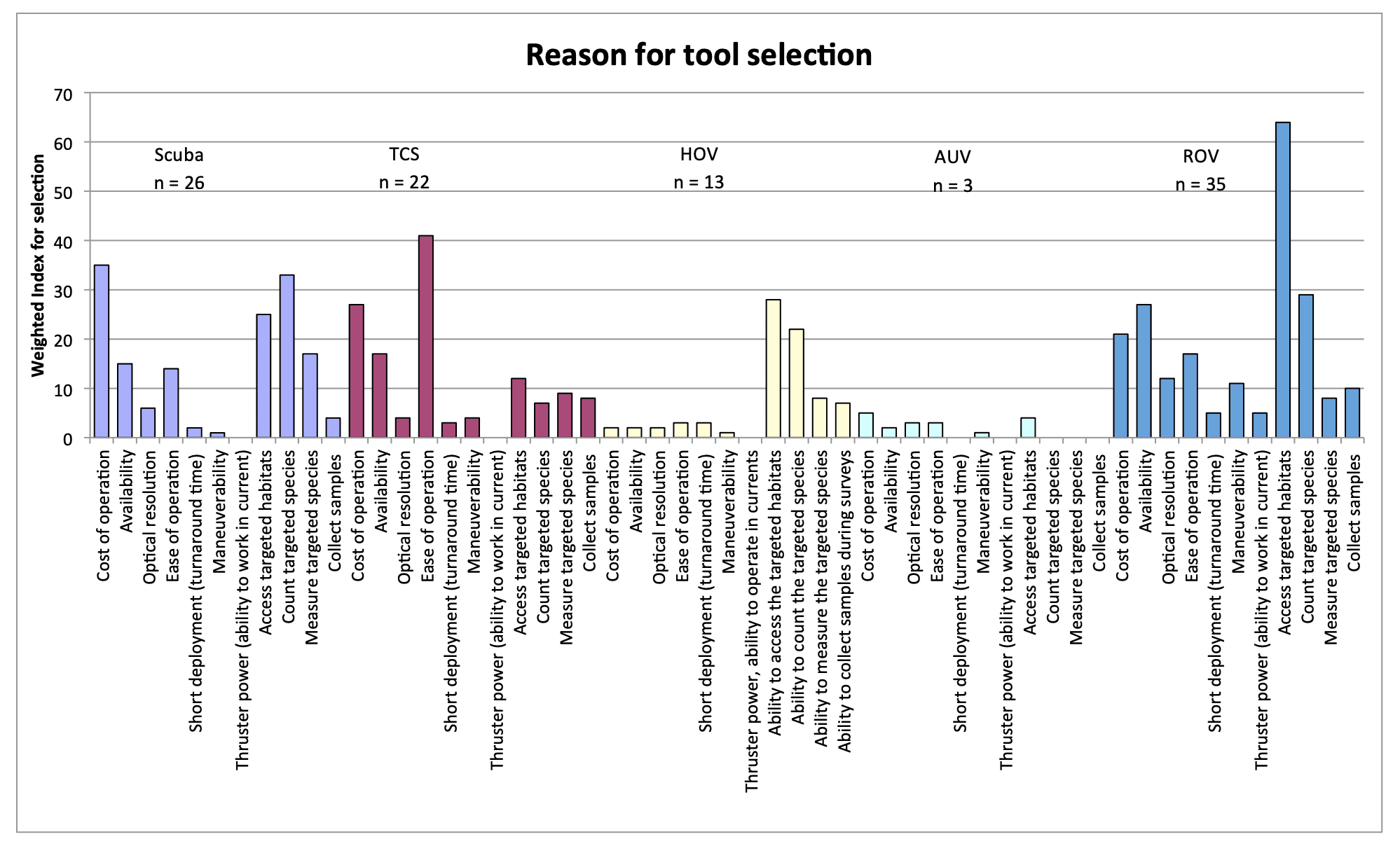
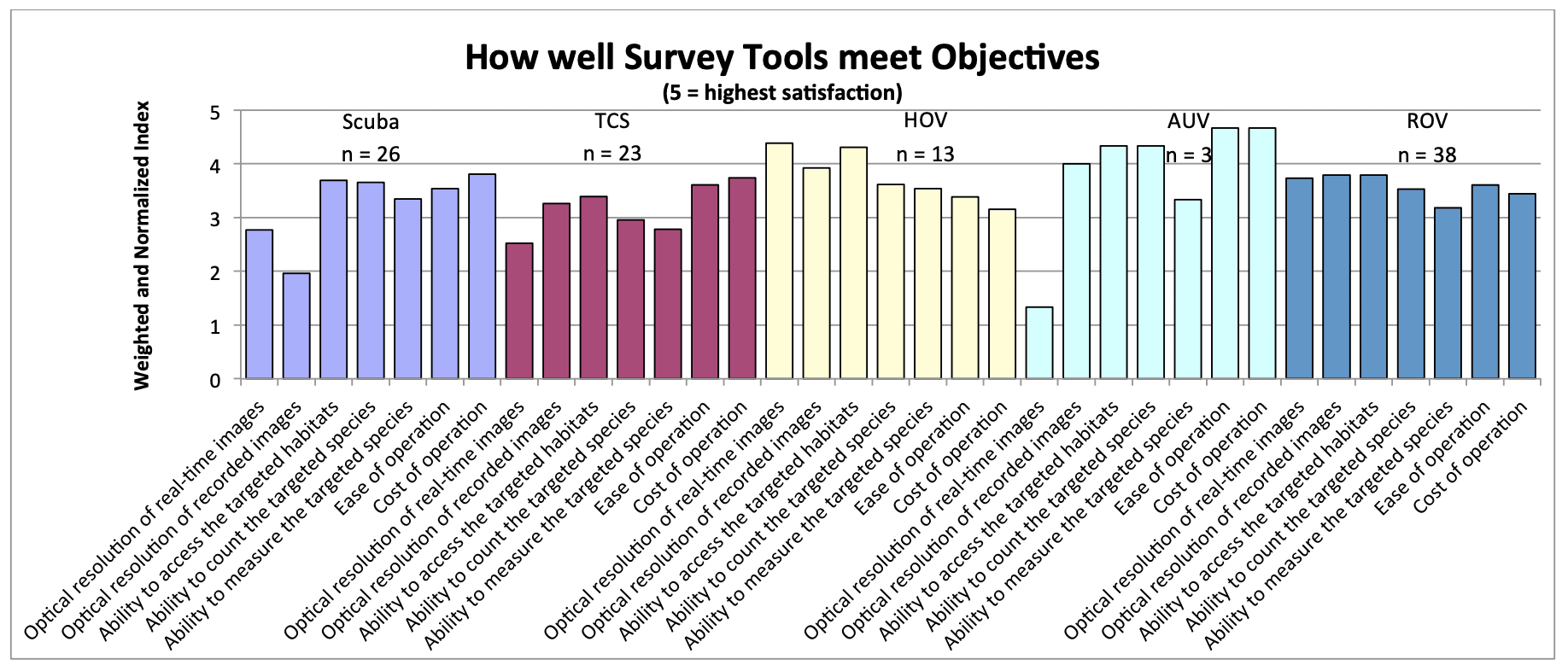
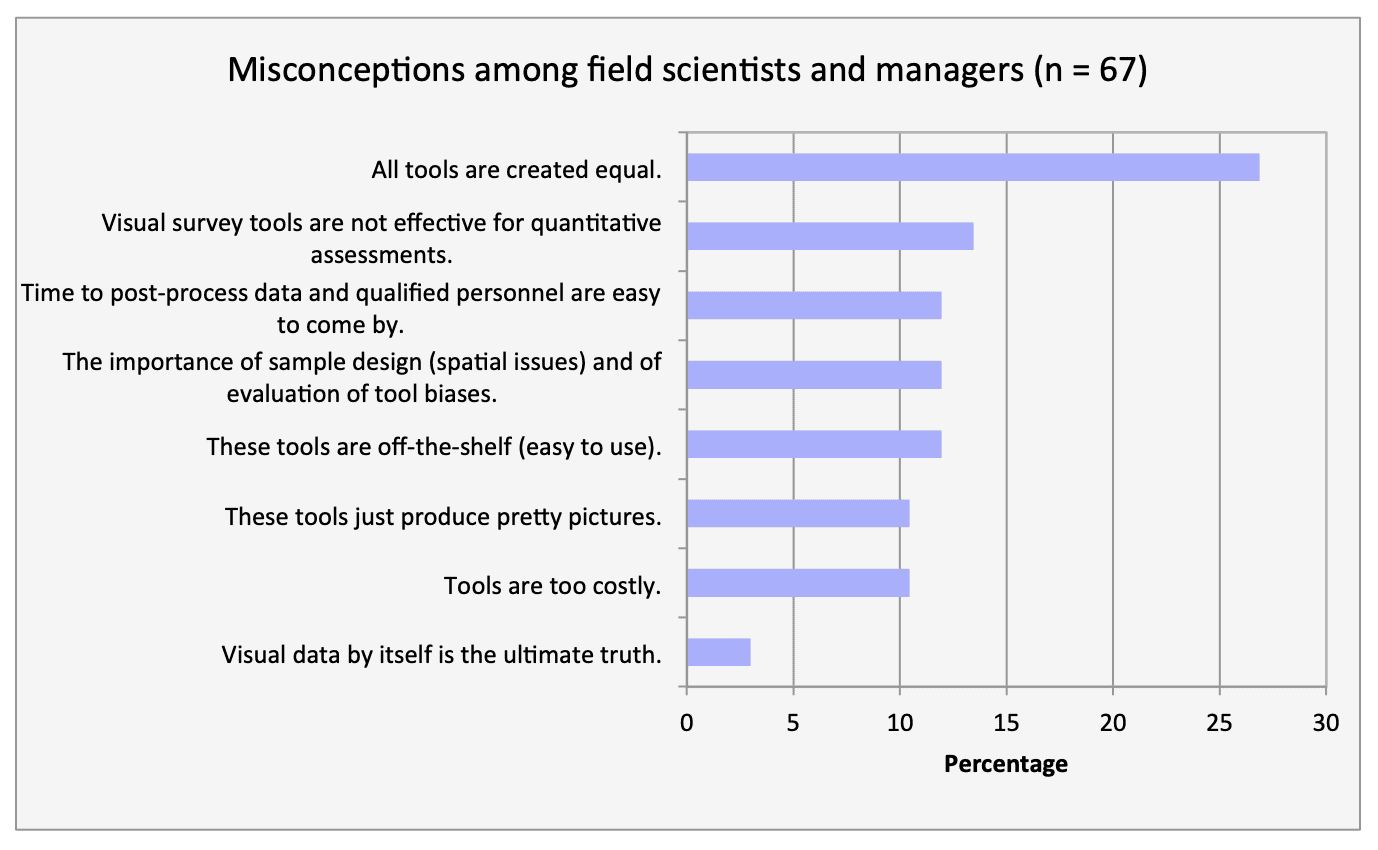
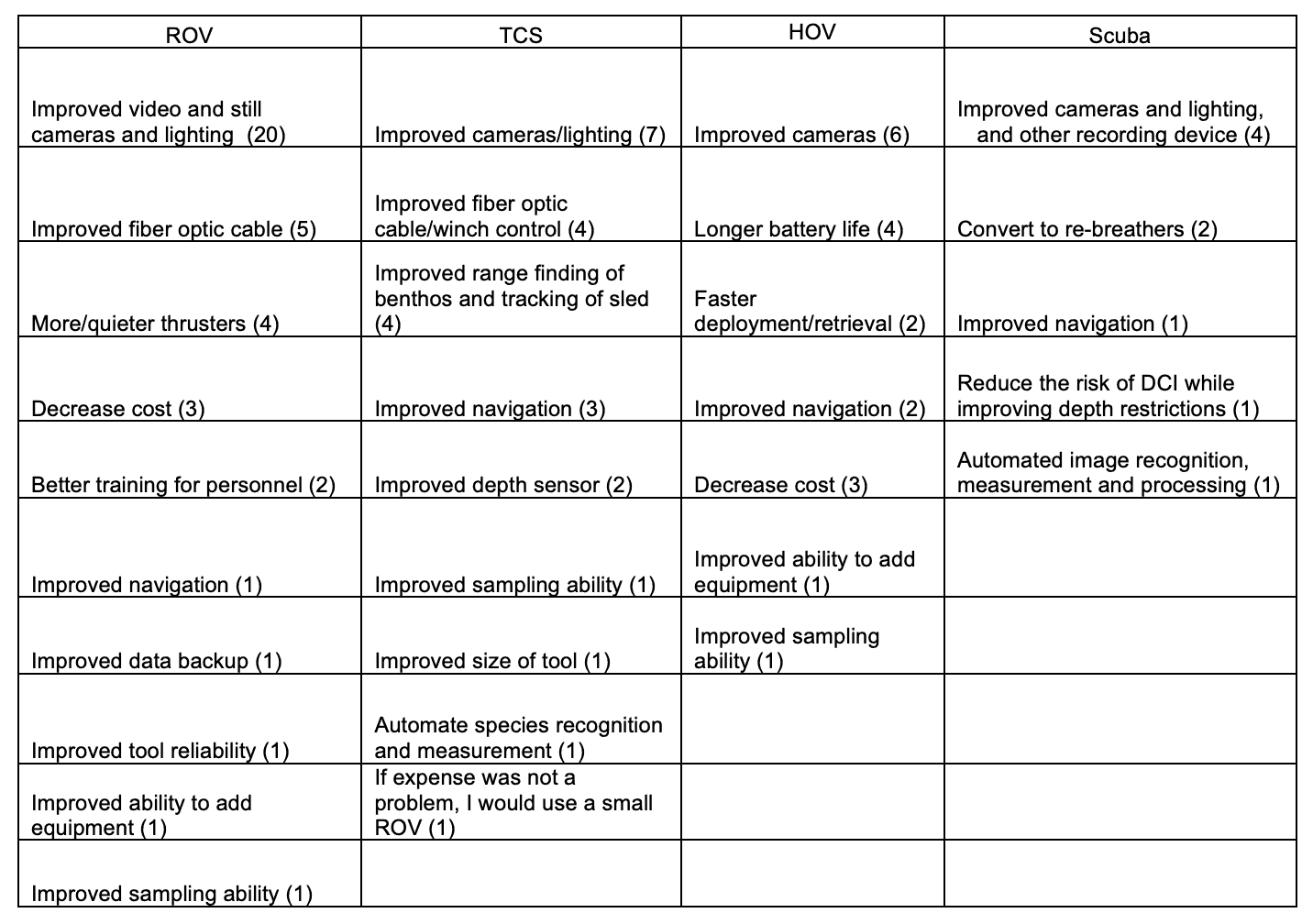
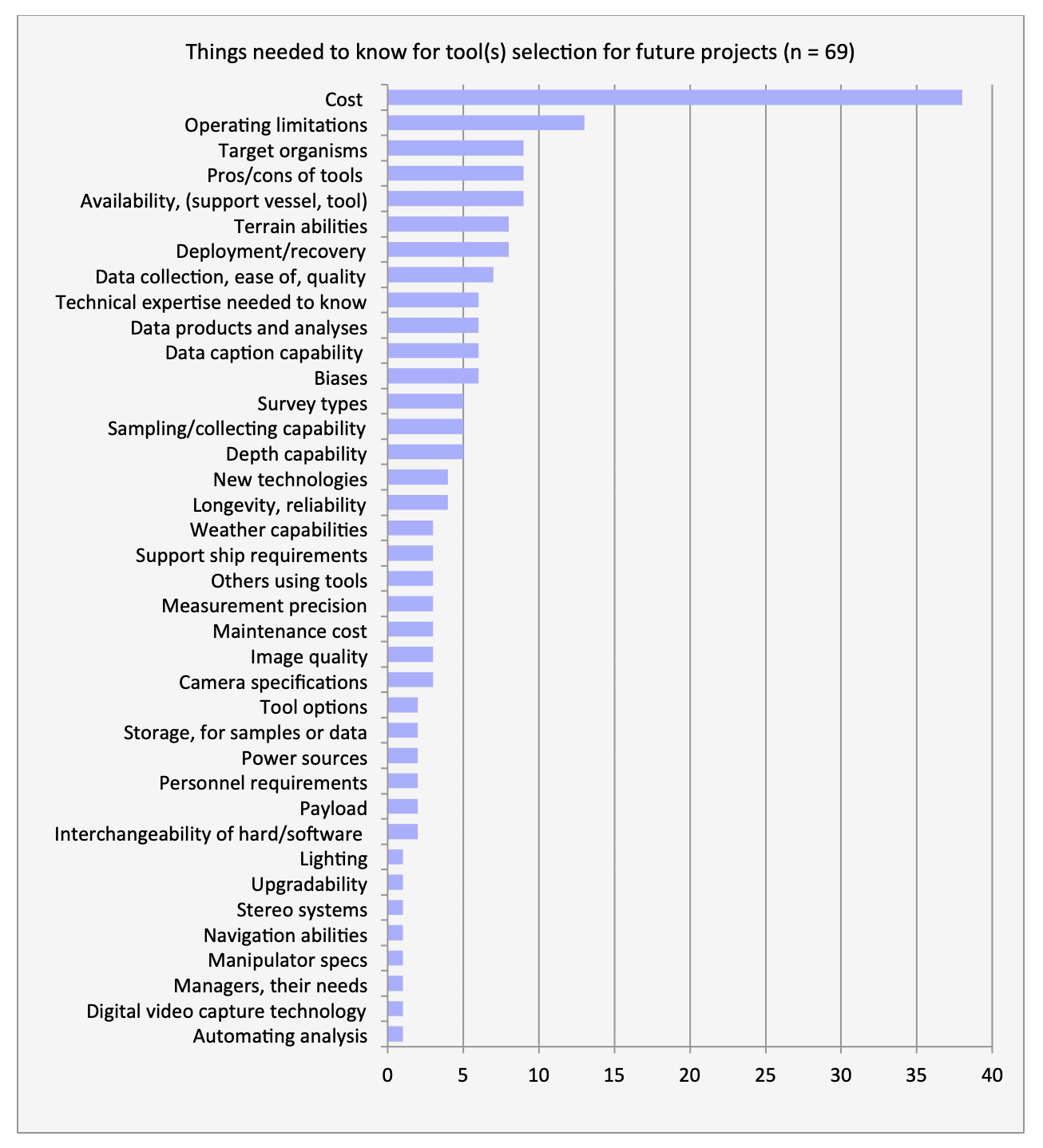
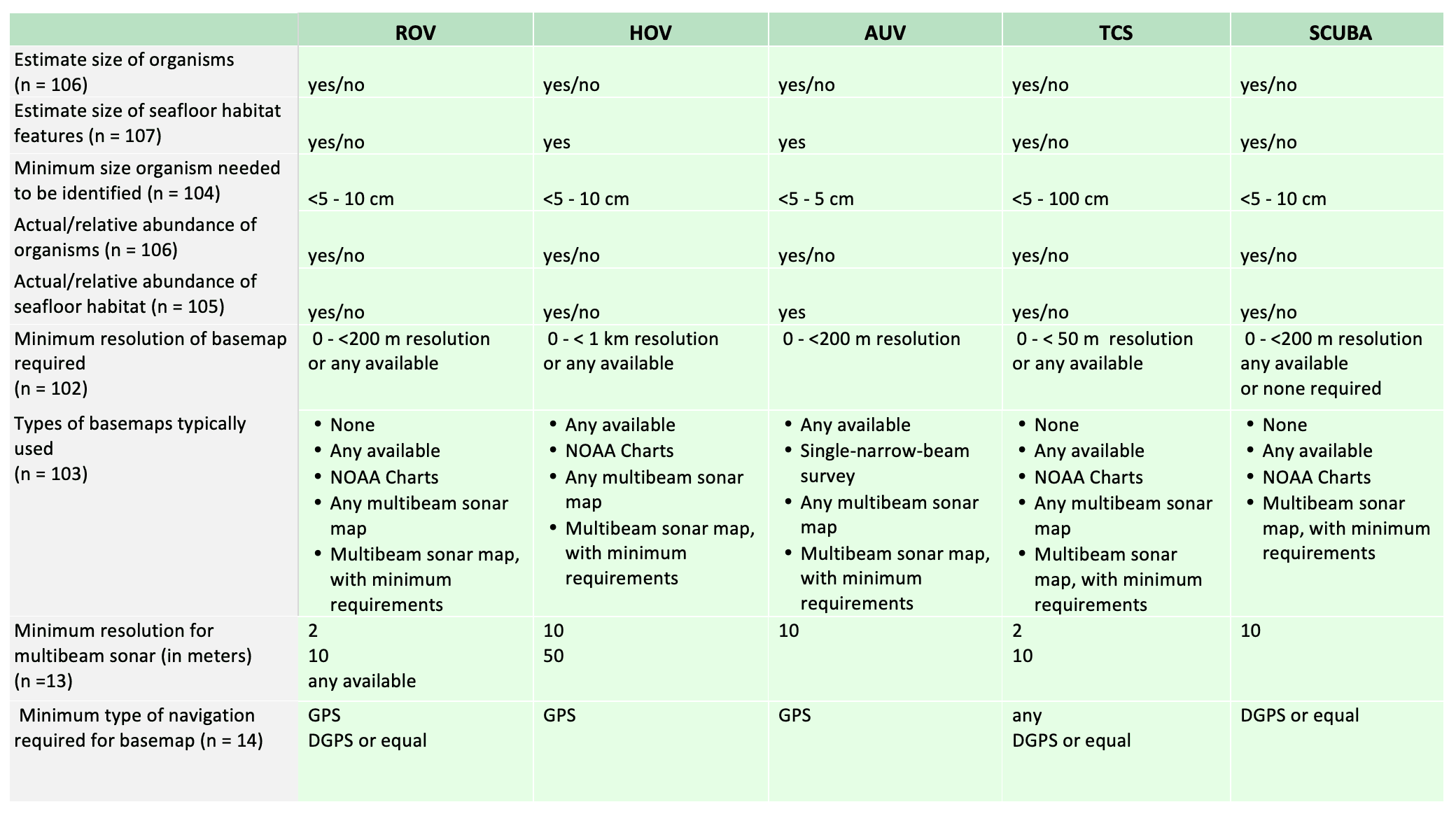
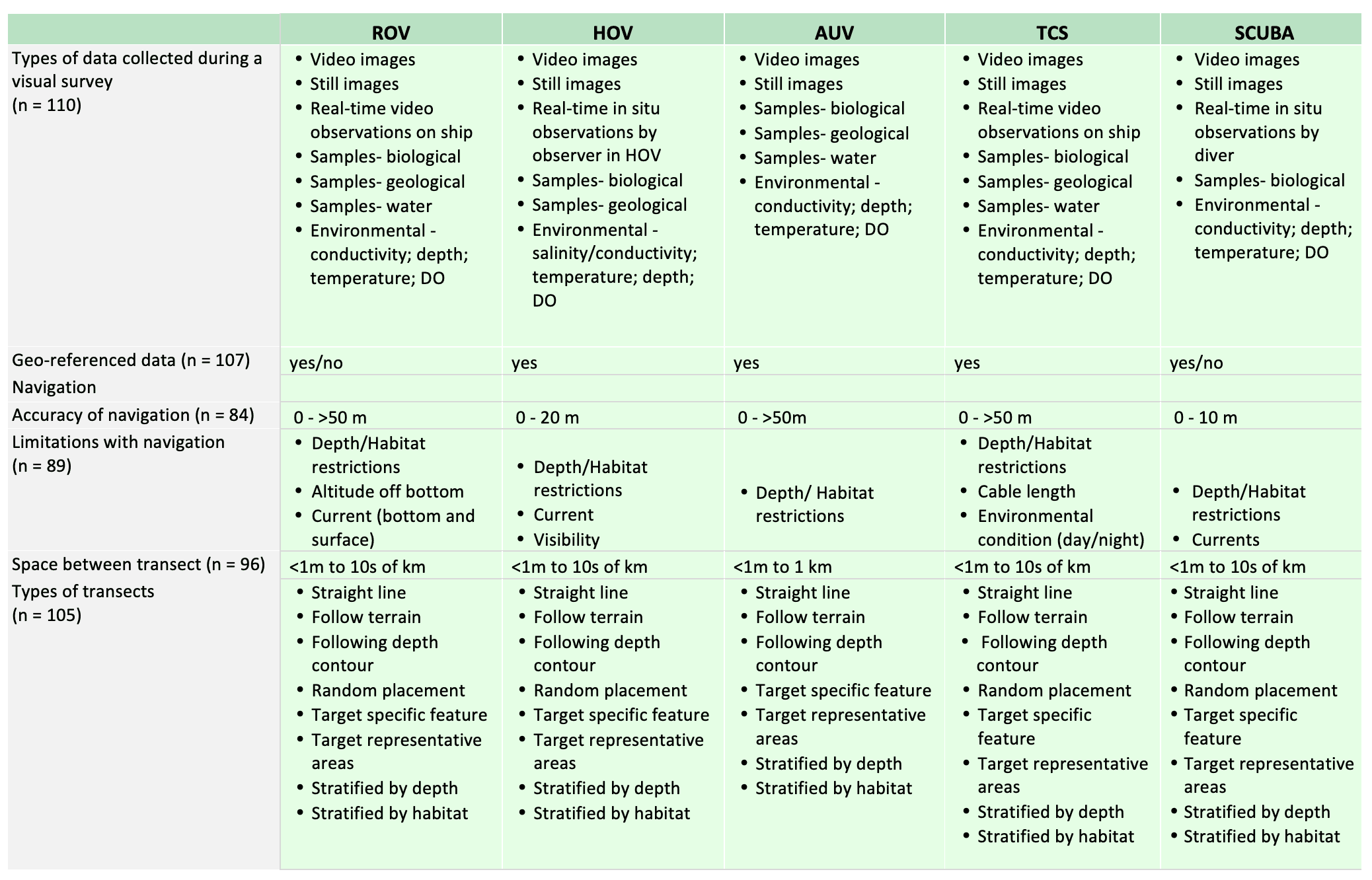
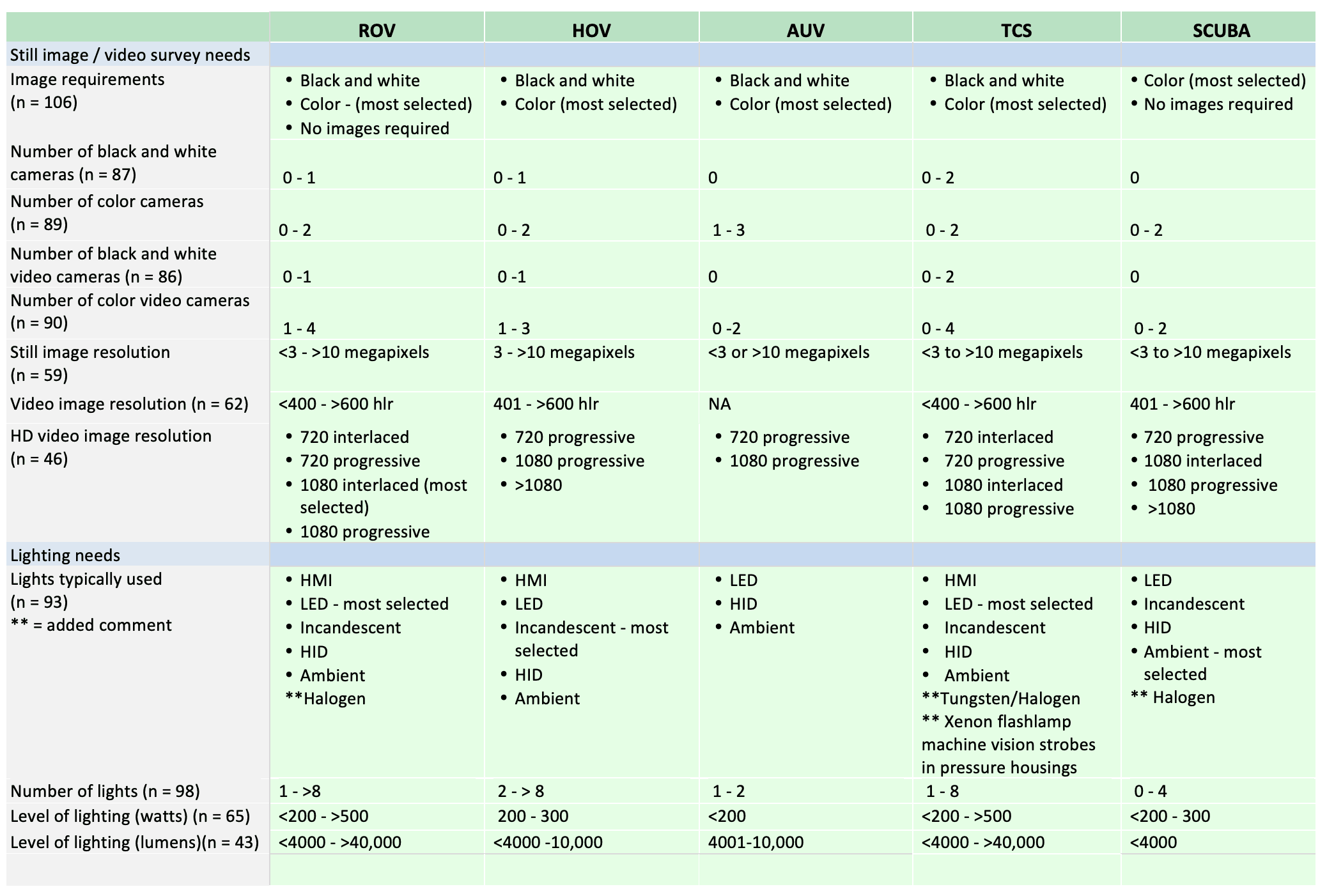
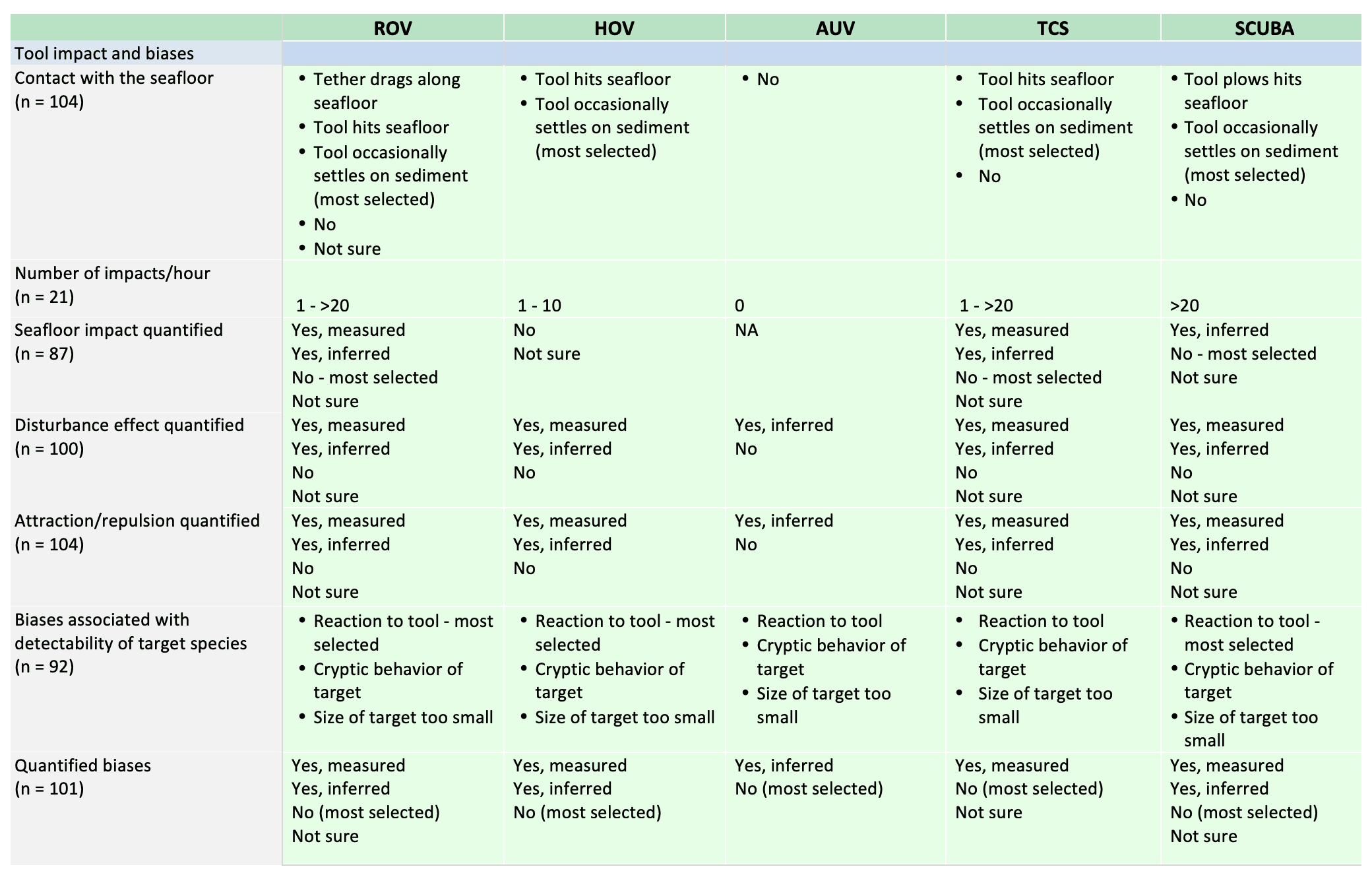
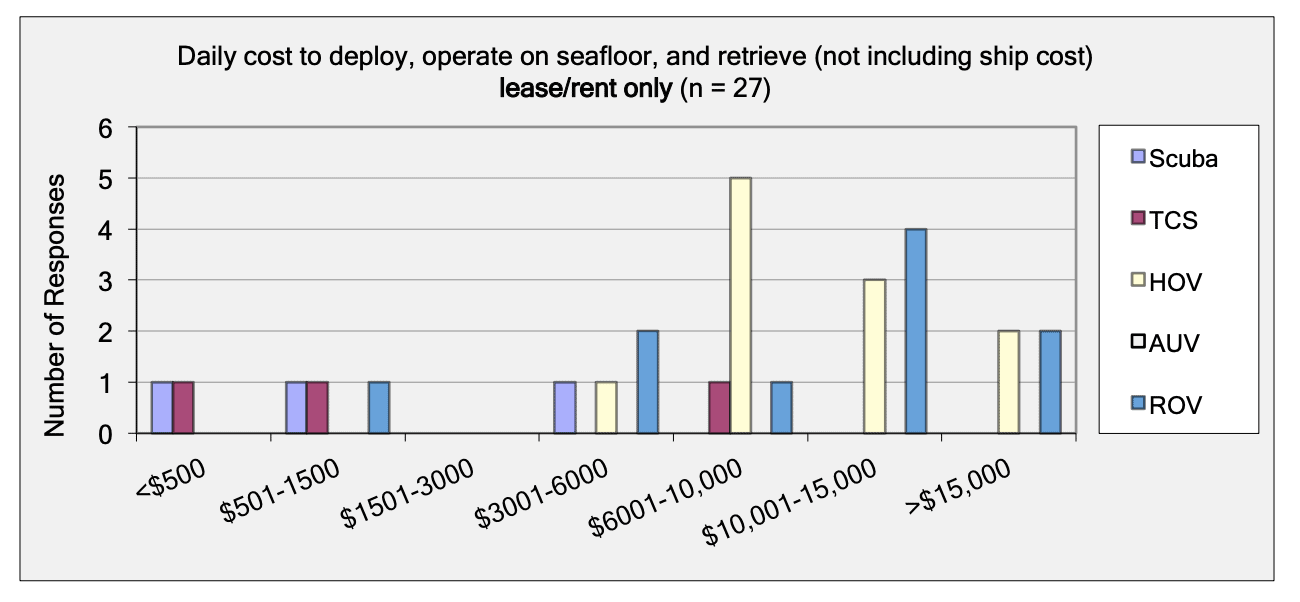 For shallow working depths it appears that the number of ROV users who own this tool equals the number of ROV users who lease/rent. For working in deeper depths (>50m) it appears that more users own, however in very deep depths (>1000m) more people lease/rent, than own.
For shallow working depths it appears that the number of ROV users who own this tool equals the number of ROV users who lease/rent. For working in deeper depths (>50m) it appears that more users own, however in very deep depths (>1000m) more people lease/rent, than own.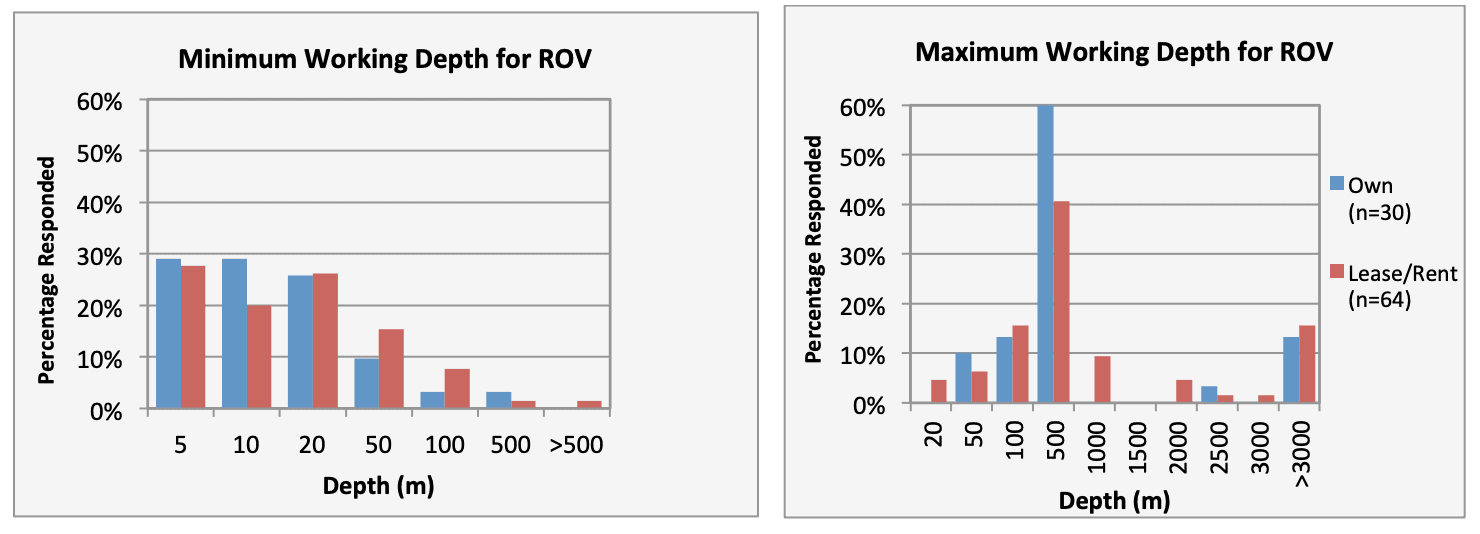
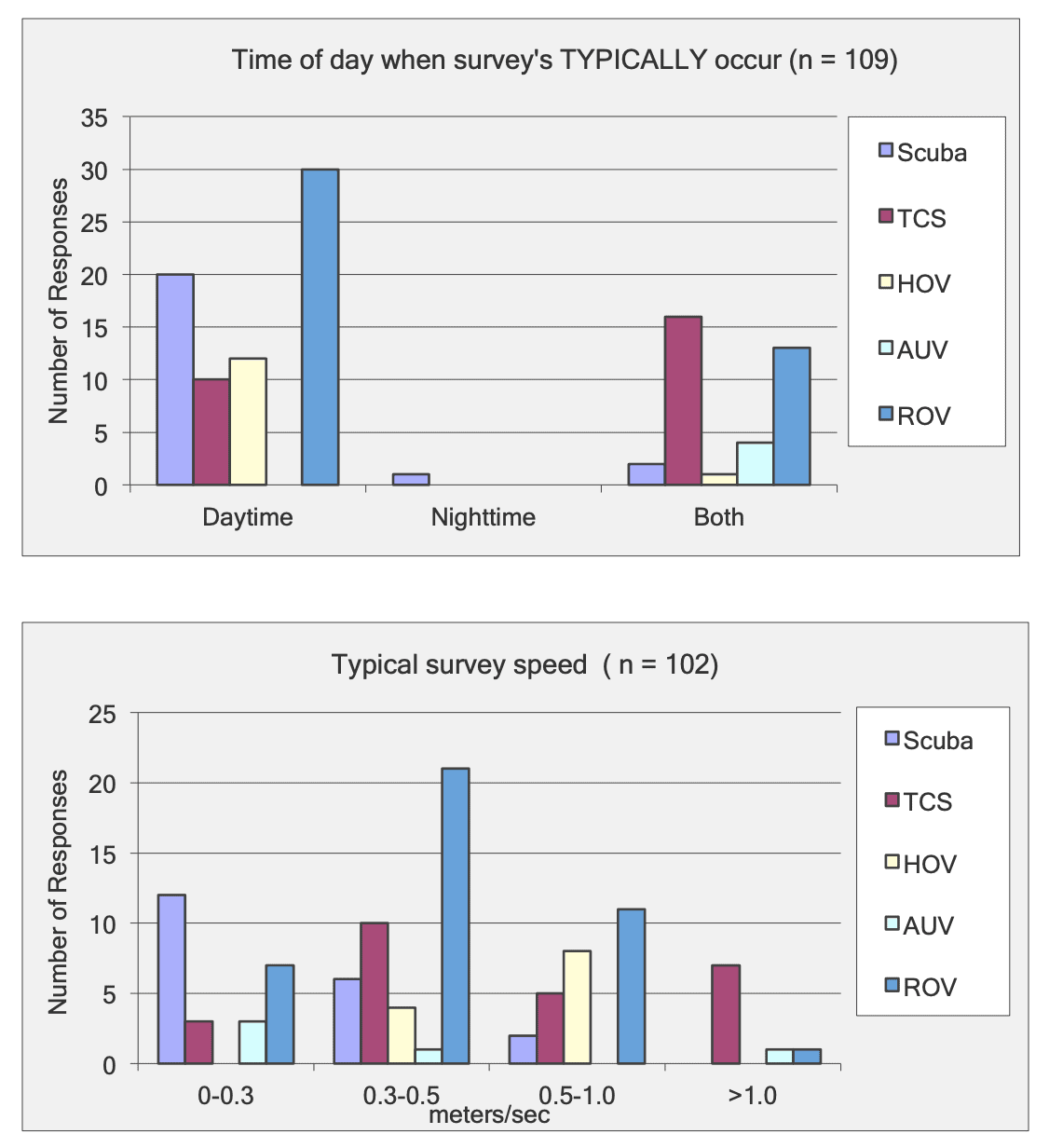
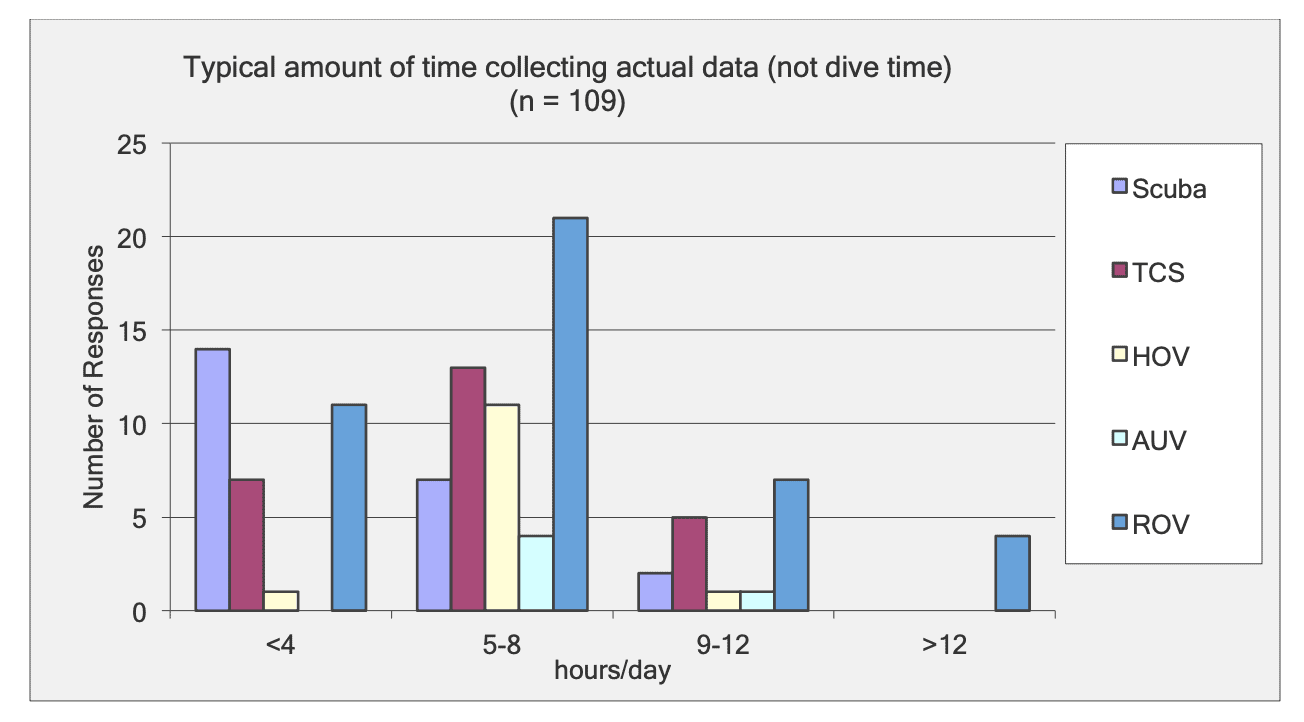
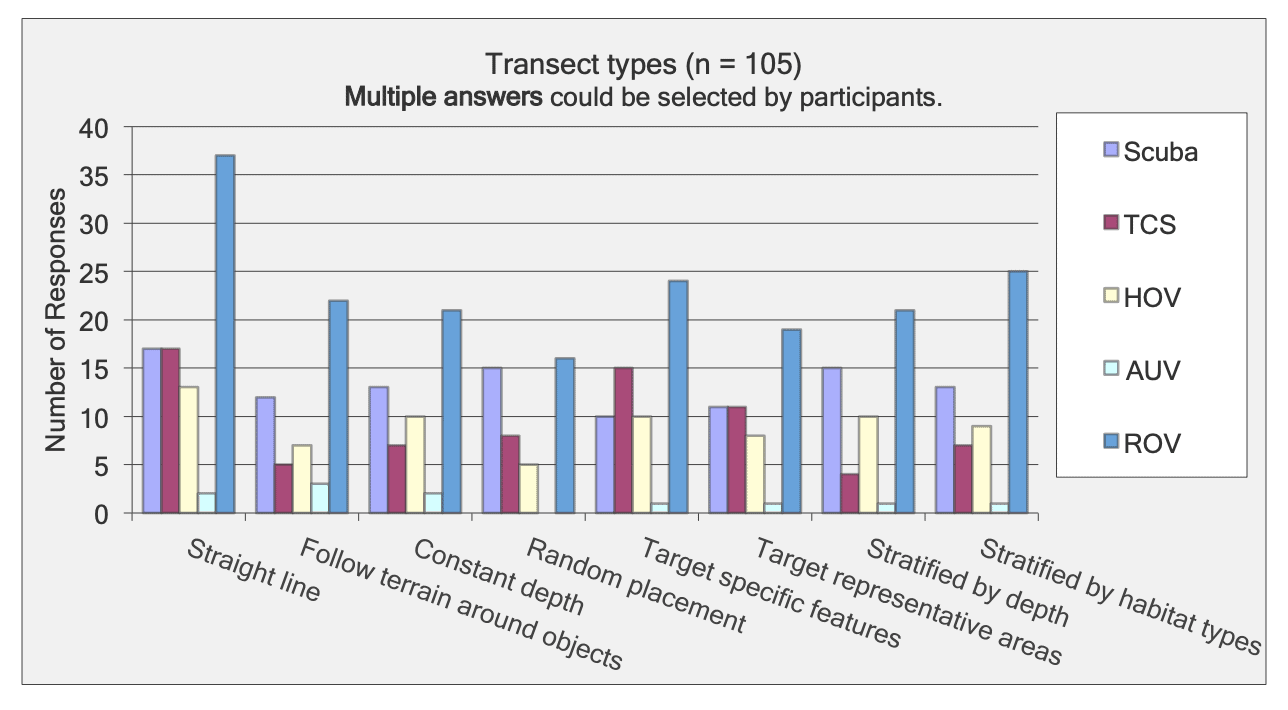


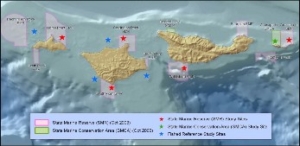
 This report summarizes the results of a multi-year study (September 2011 – January 2015) to characterize mid-depth rocky reef and soft bottom ecosystems in the California Marine Life Protection Act’s South Coast (SC) Study Region. Our specific objective was to characterize the seafloor habitats and associated biological communities within and adjacent to the State Marine Reserves (SMRs) and Conservation Areas (SMCAs) at the time of implementation.
This report summarizes the results of a multi-year study (September 2011 – January 2015) to characterize mid-depth rocky reef and soft bottom ecosystems in the California Marine Life Protection Act’s South Coast (SC) Study Region. Our specific objective was to characterize the seafloor habitats and associated biological communities within and adjacent to the State Marine Reserves (SMRs) and Conservation Areas (SMCAs) at the time of implementation.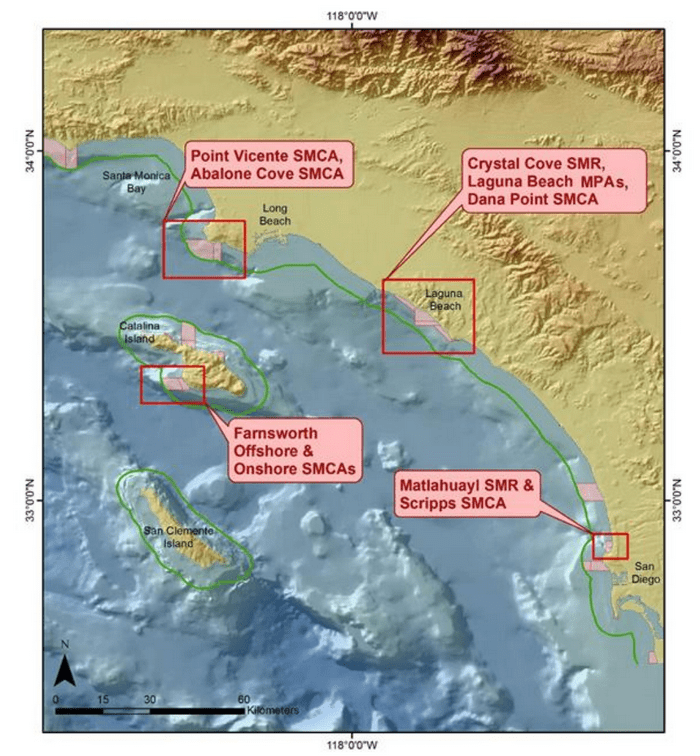
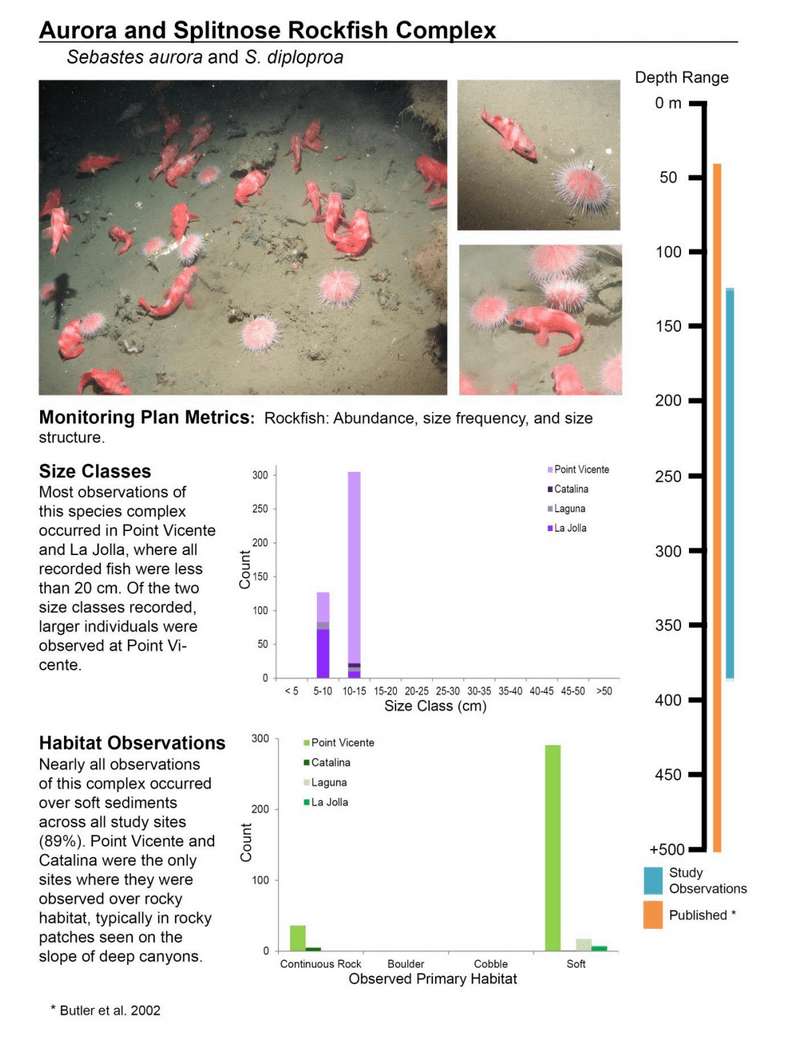
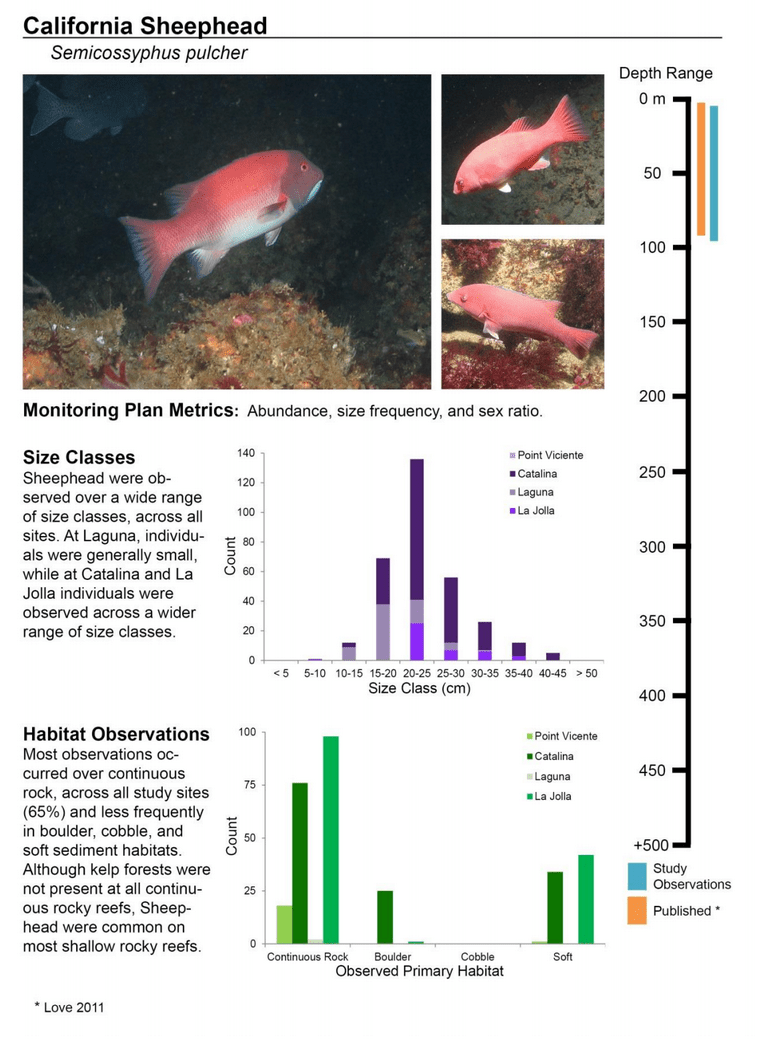
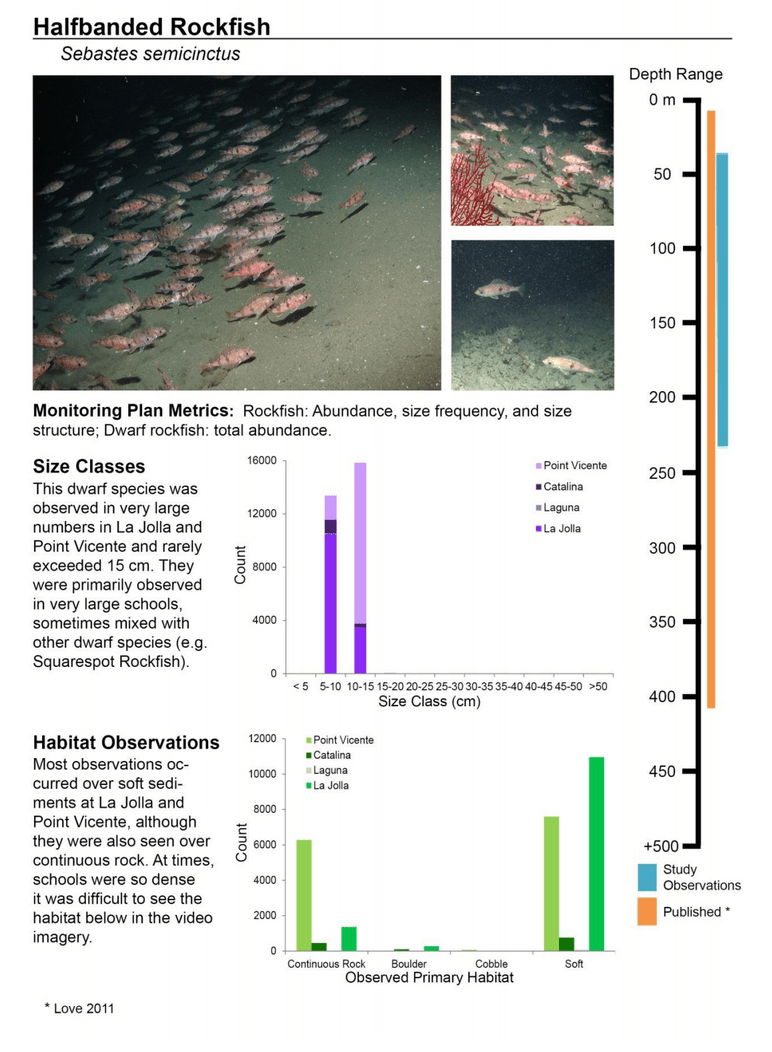
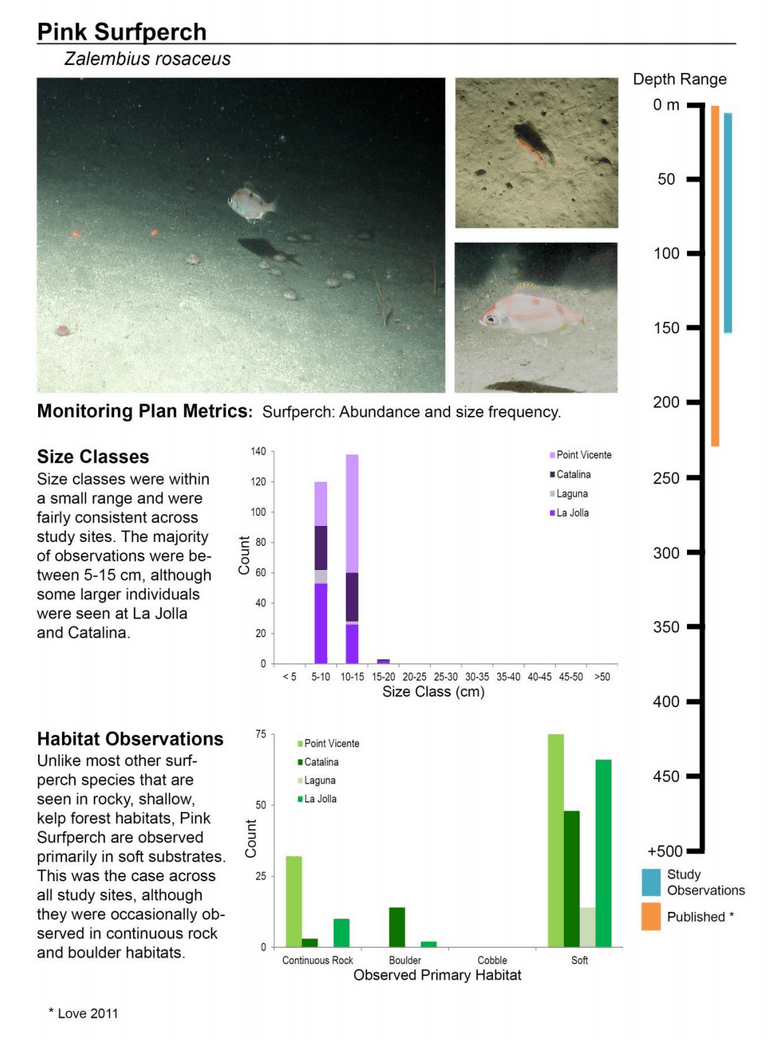
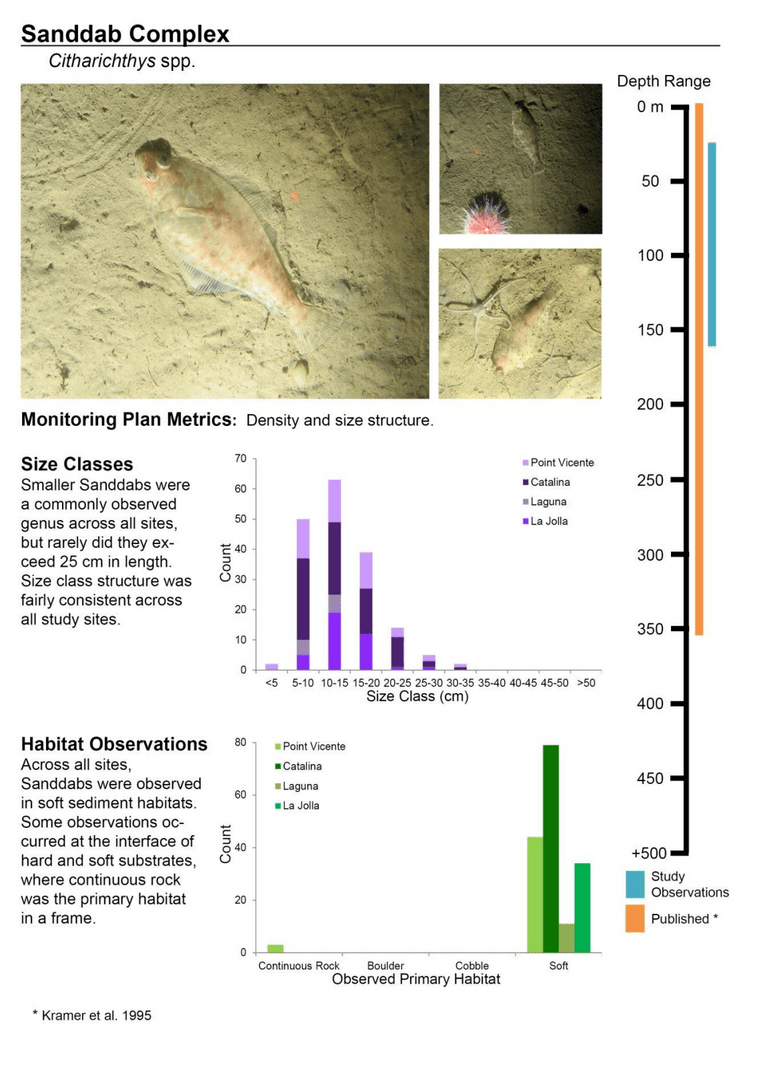
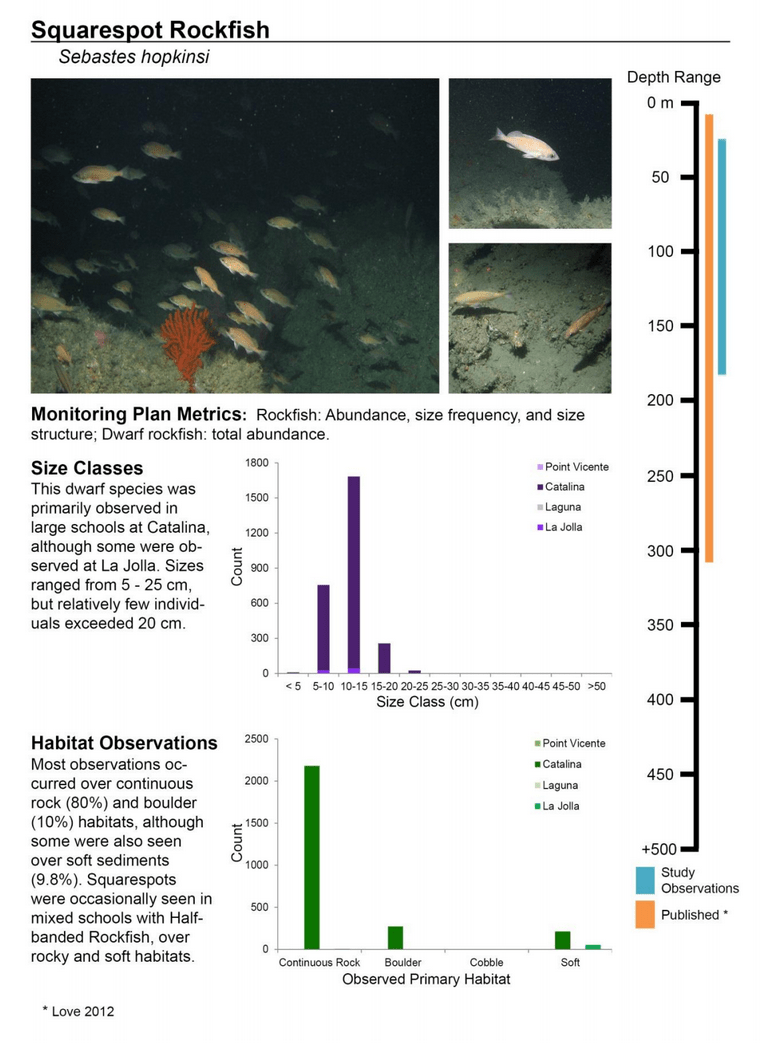
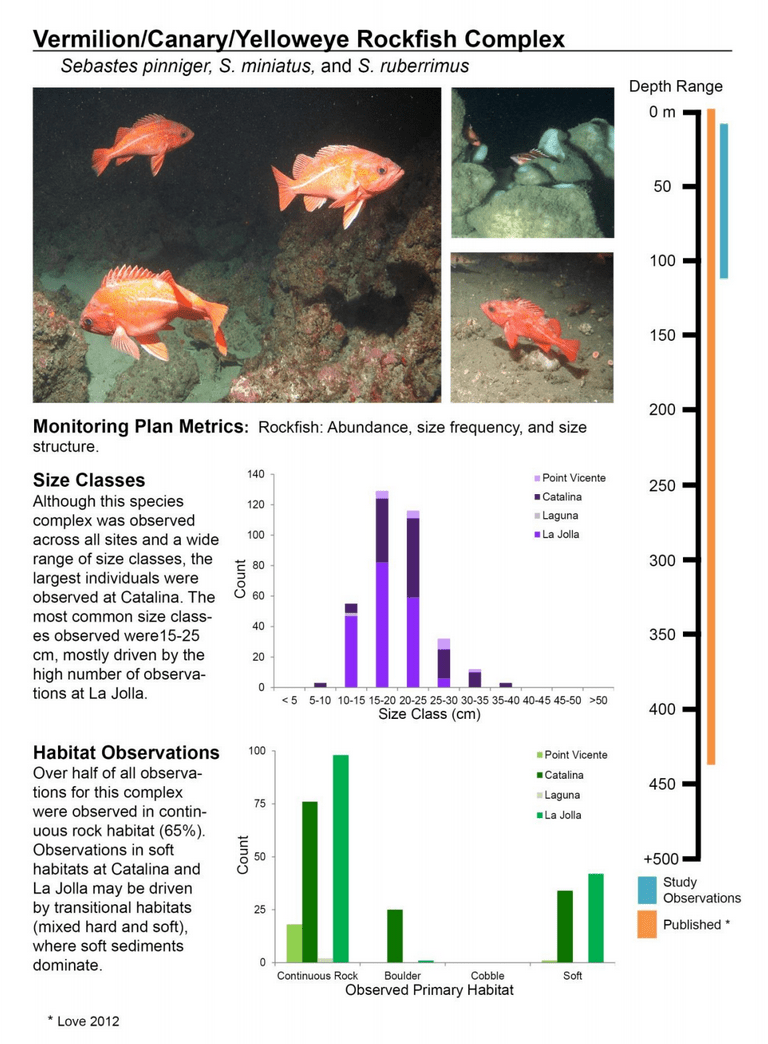
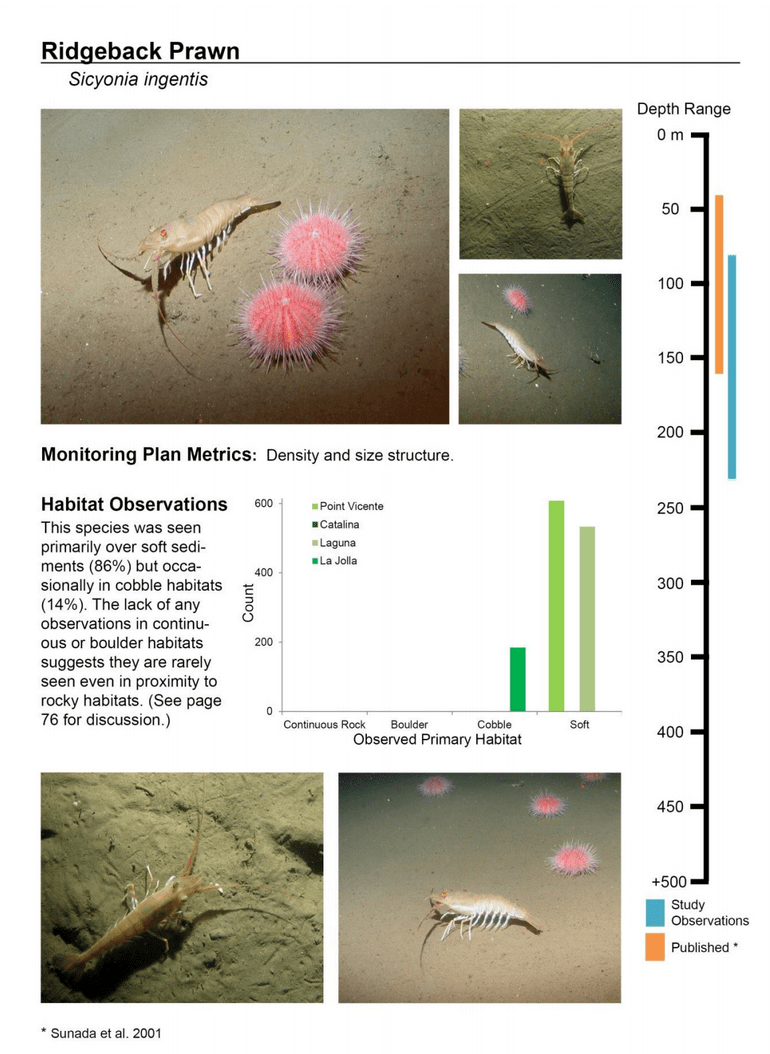
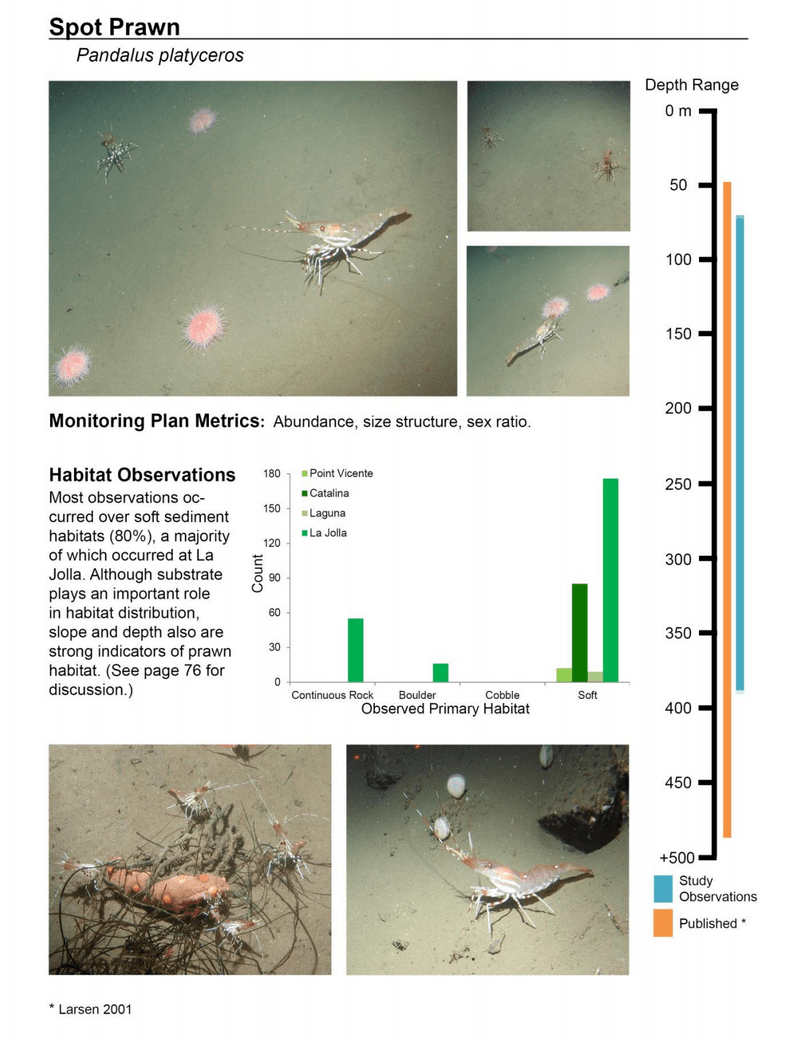
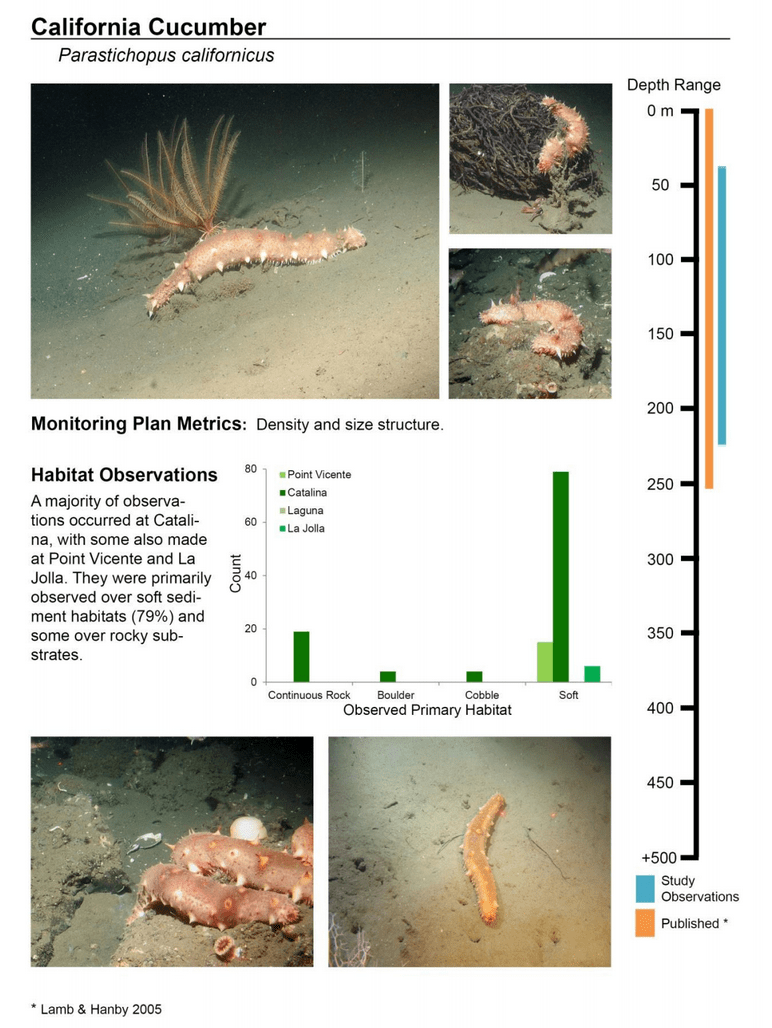
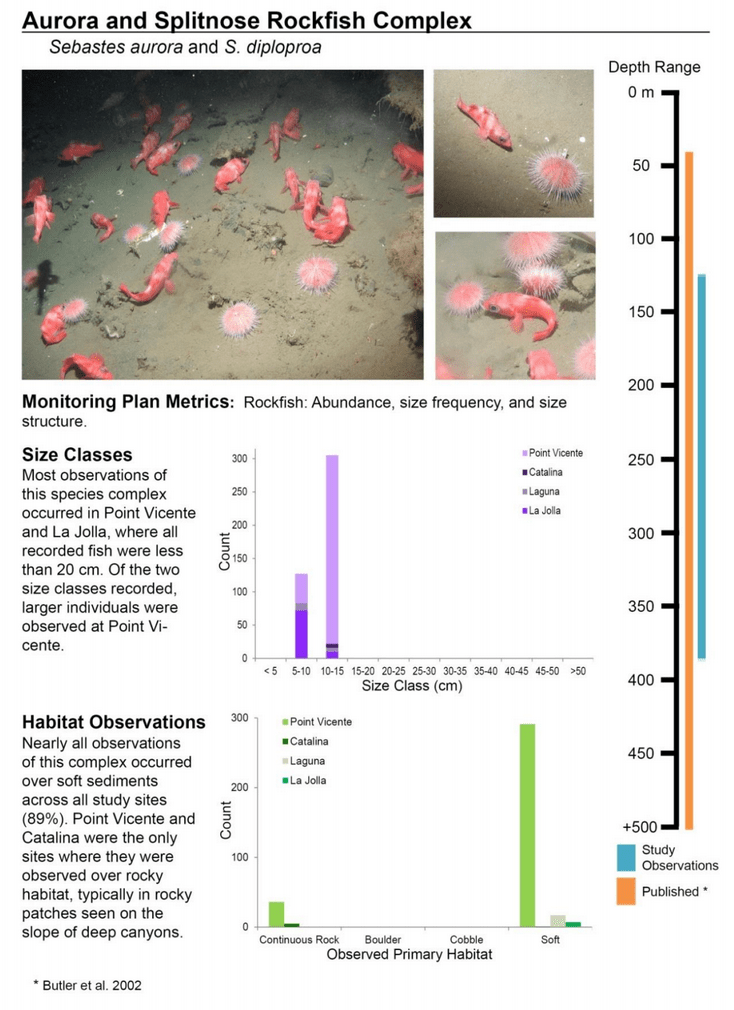
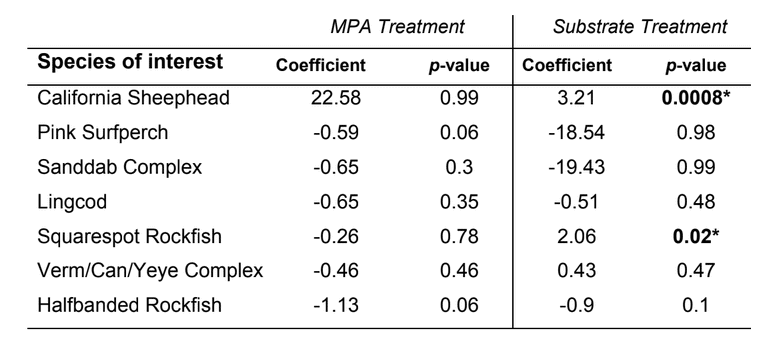
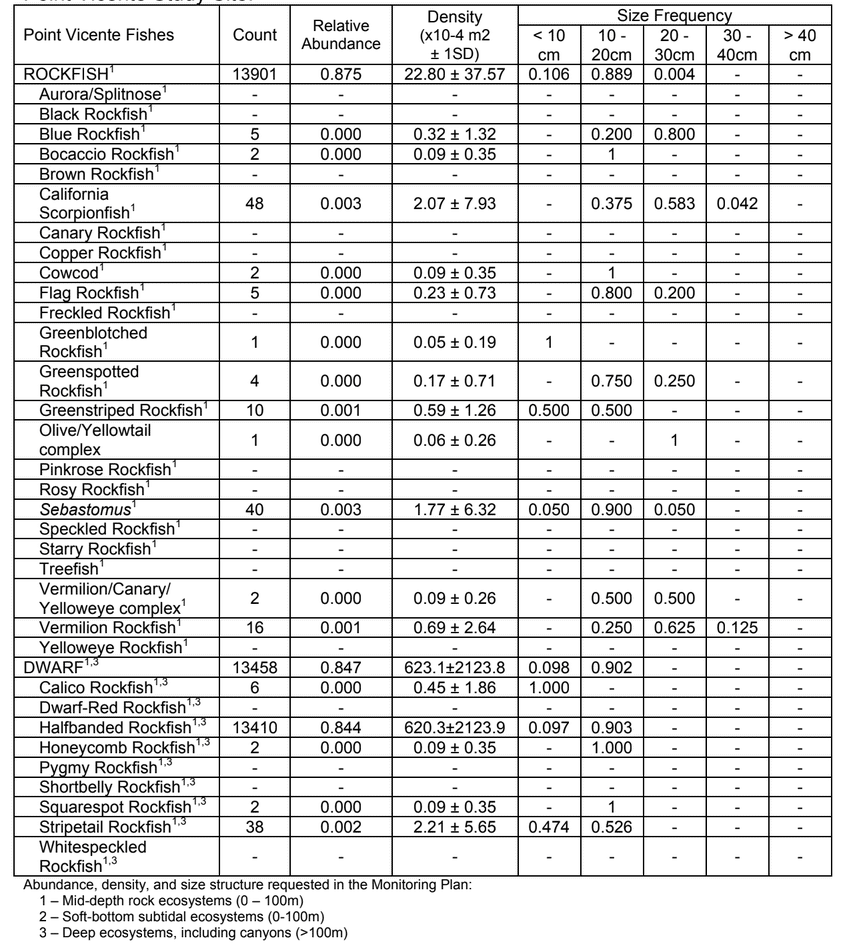
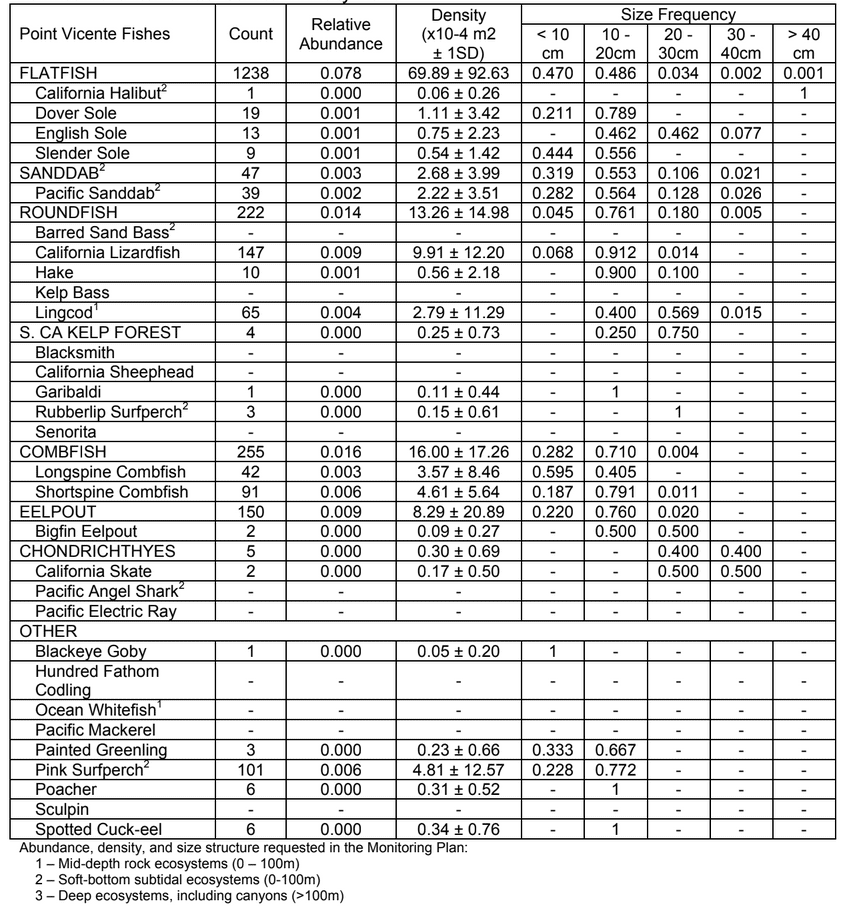
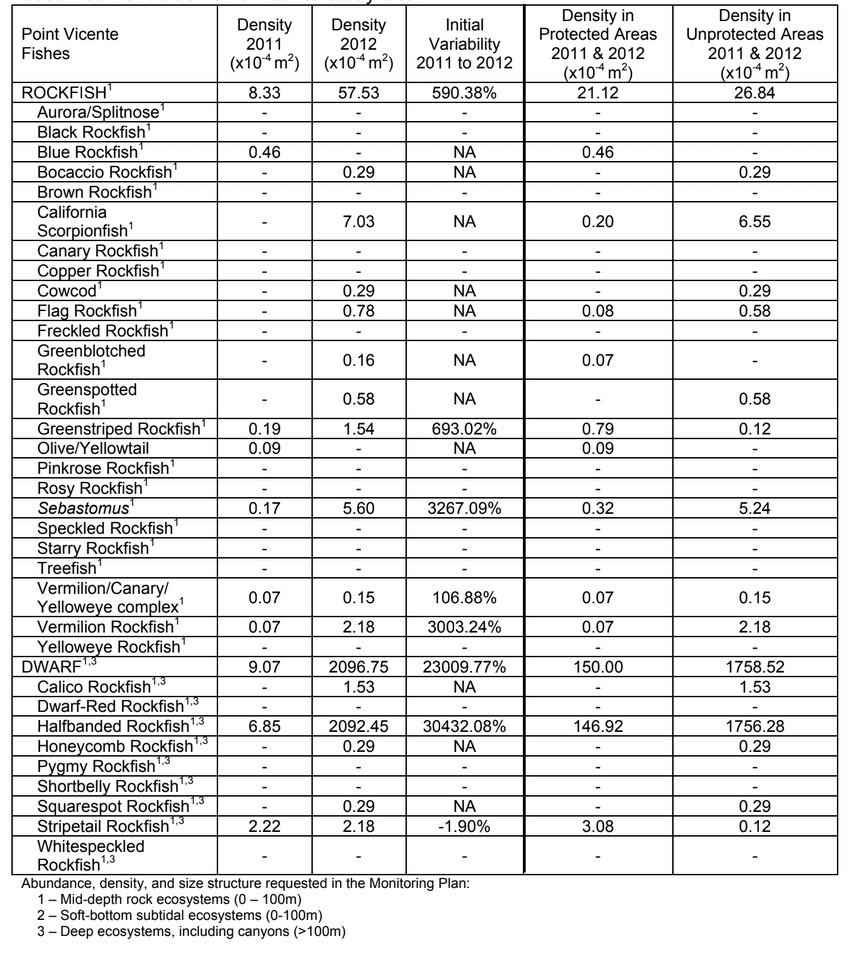
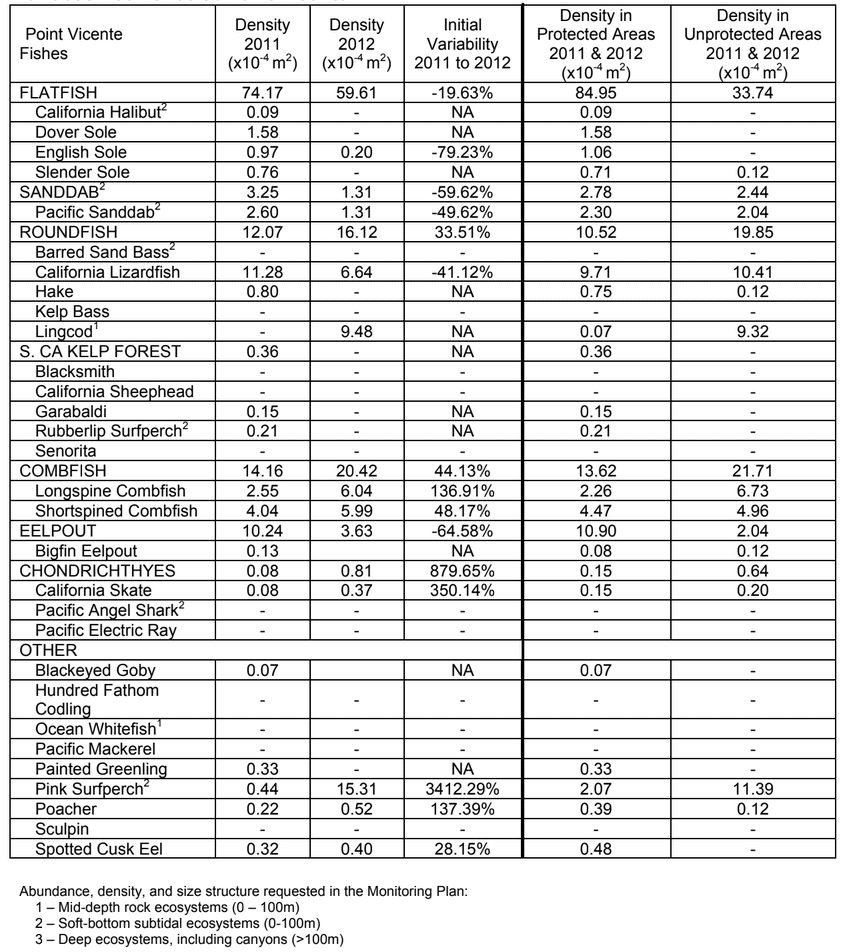
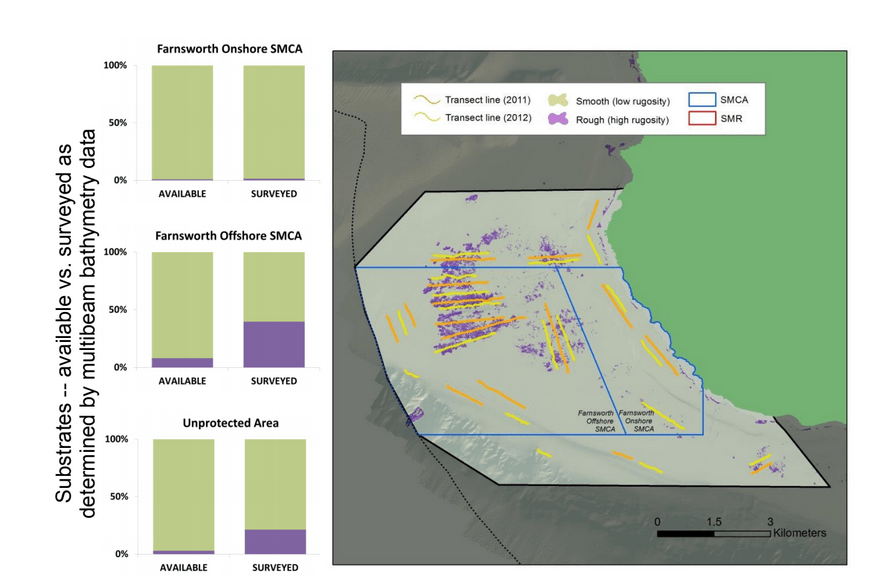
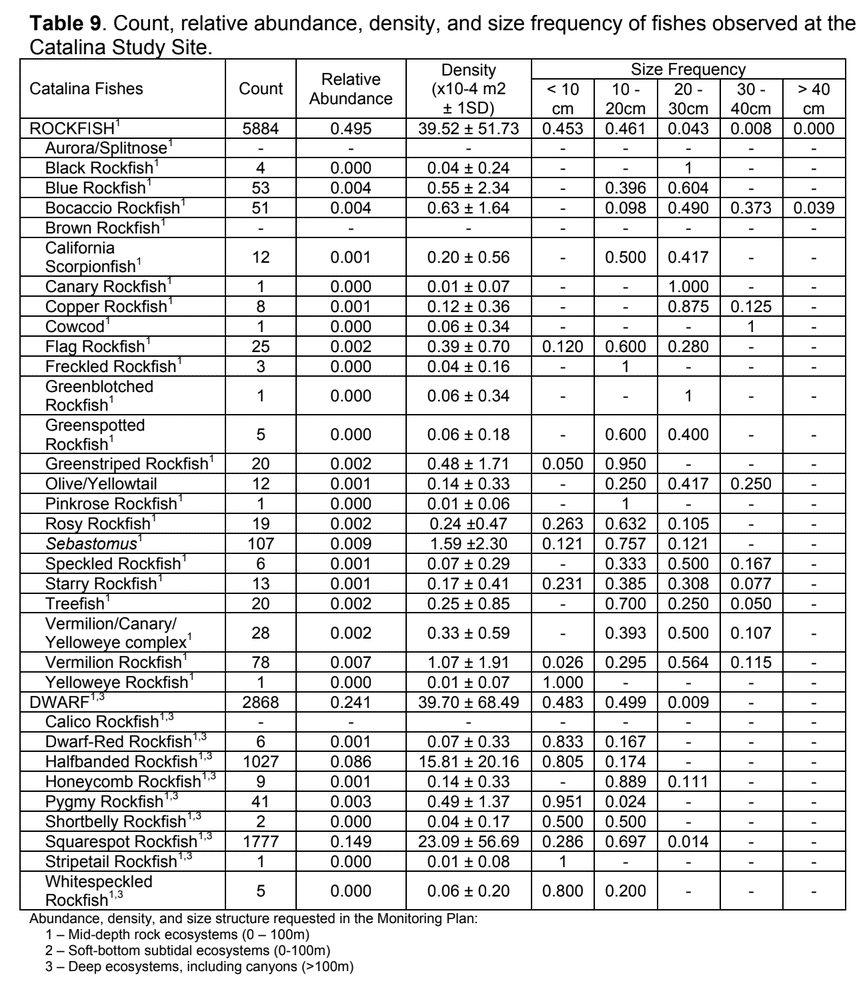
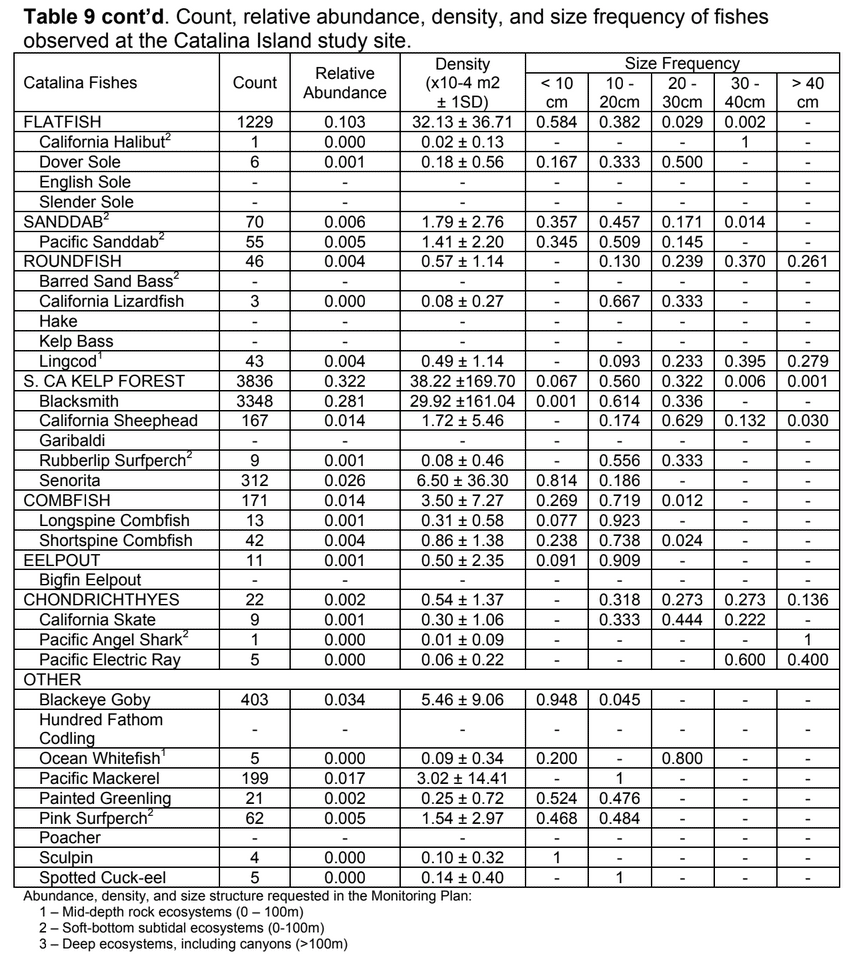
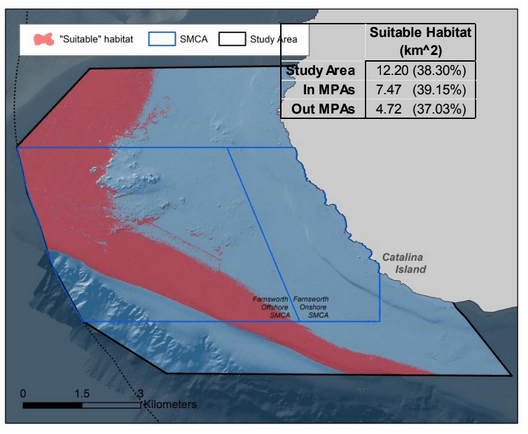
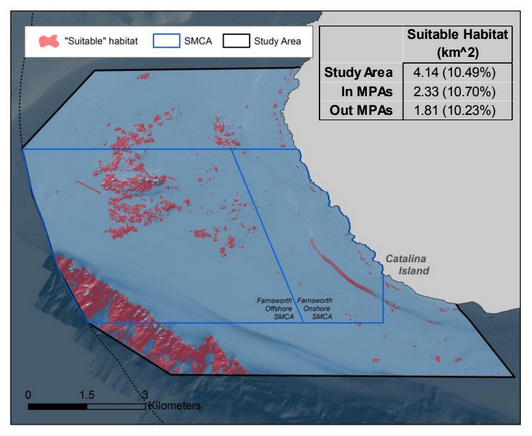
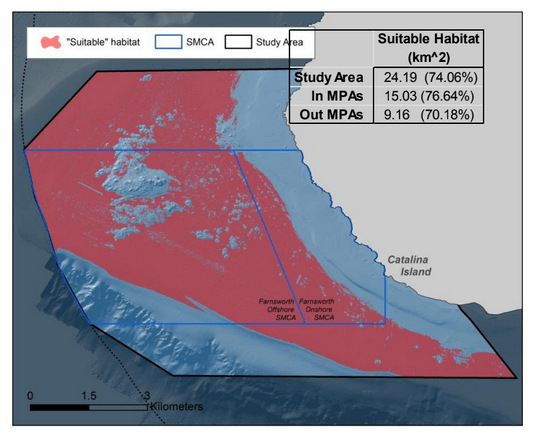
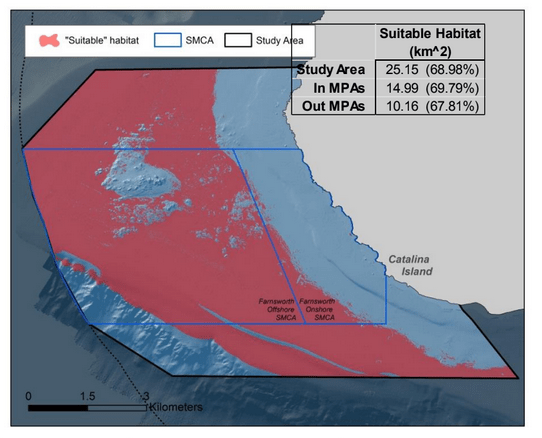
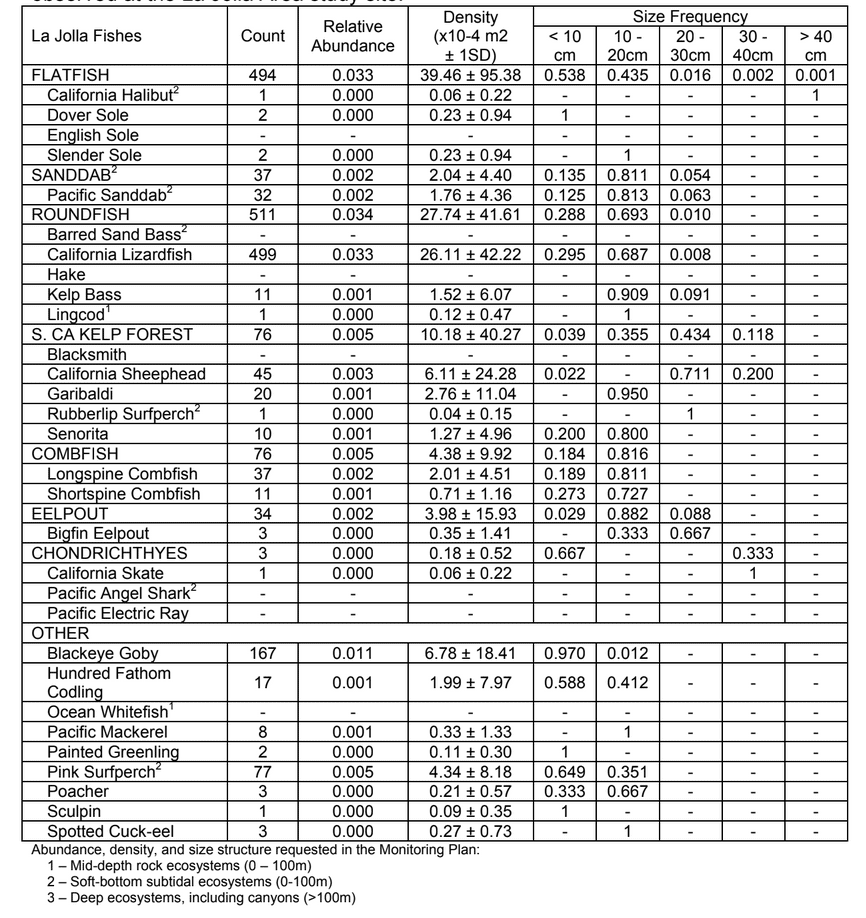
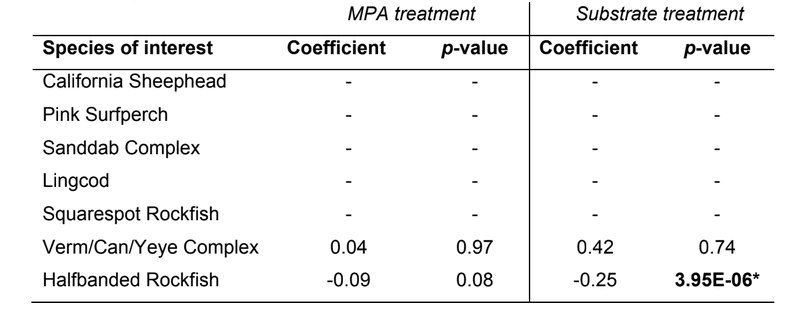
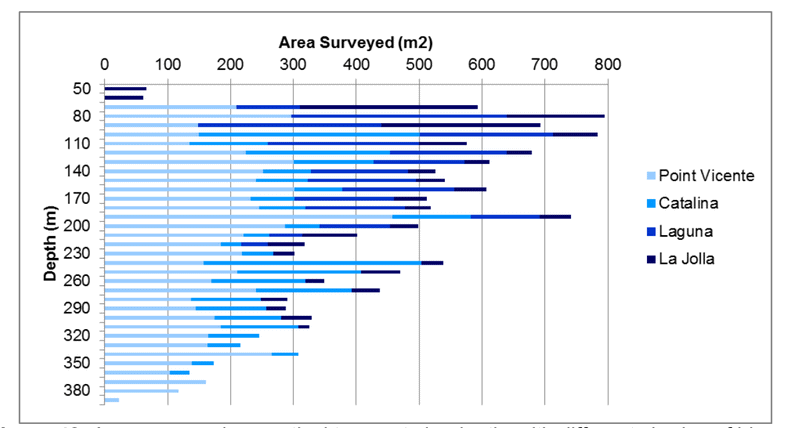
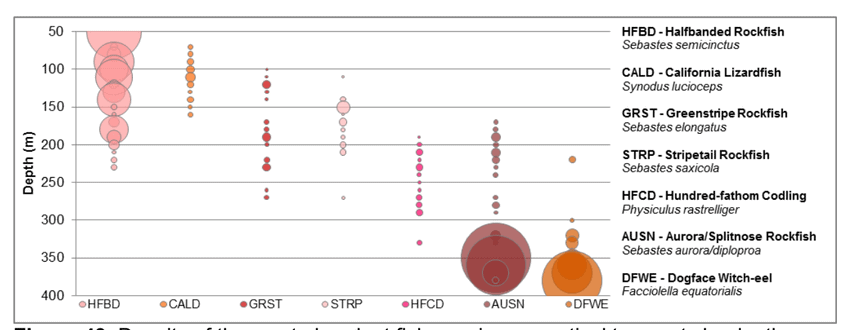
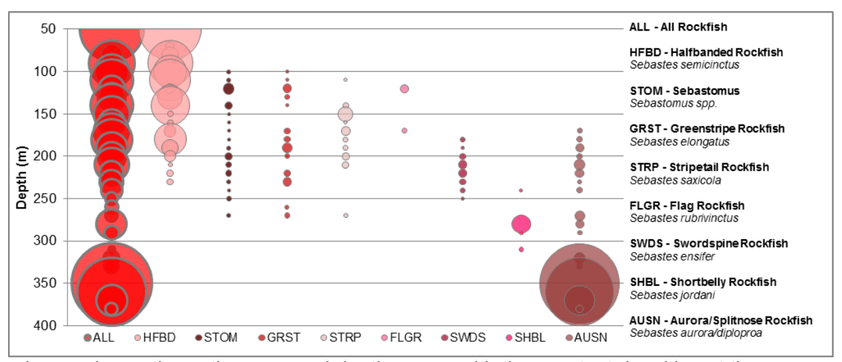
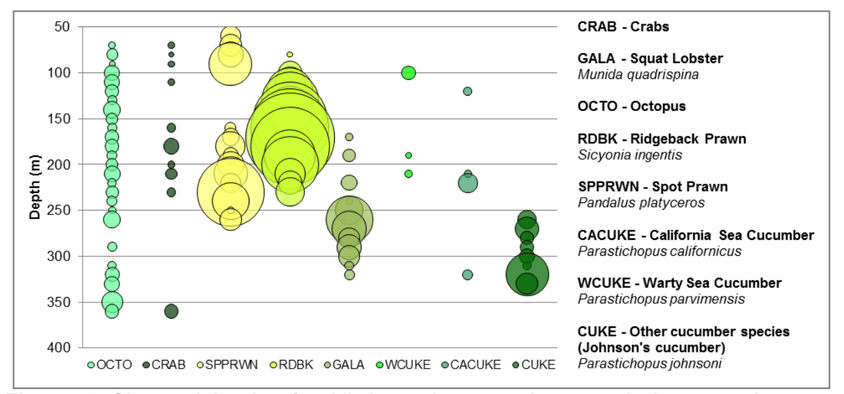
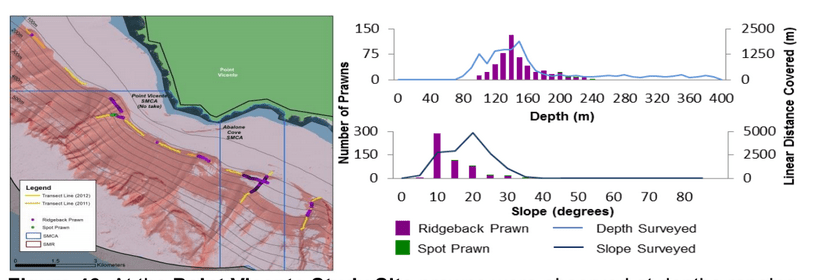
 to rocky reefs, and the transitional areas in between. At the northernmost mainland site (Point Vicente), Halfbanded Rockfish were the most abundant of the 16,853 fish we observed. It is important to note that we were prevented from sampling the limited rocky reef areas along the mainland due to the significant entanglement hazards created by Giant Kelp (Macrocystis pyrifera) and lobster pots. In the south (La Jolla), which included both shelf sites as well as sites deep within the submarine canyons, Halfbanded Rockfish also dominated the 16,867 fish observed, despite very challenging sampling conditions. Indeed, to account for the great difficulties we encountered sampling the deep submarine canyons, we developed a new sampling protocol described below in the section on Analytical products derived from baseline data. Of the 15,837 fish observed at the Farnsworth Bank MPAs along the southwest coast of Catalina Island, where visibility was generally excellent, Blacksmith were the most numerous (n=3,458). We also observed thousands of invertebrates, both mobile and sessile, across the study area.
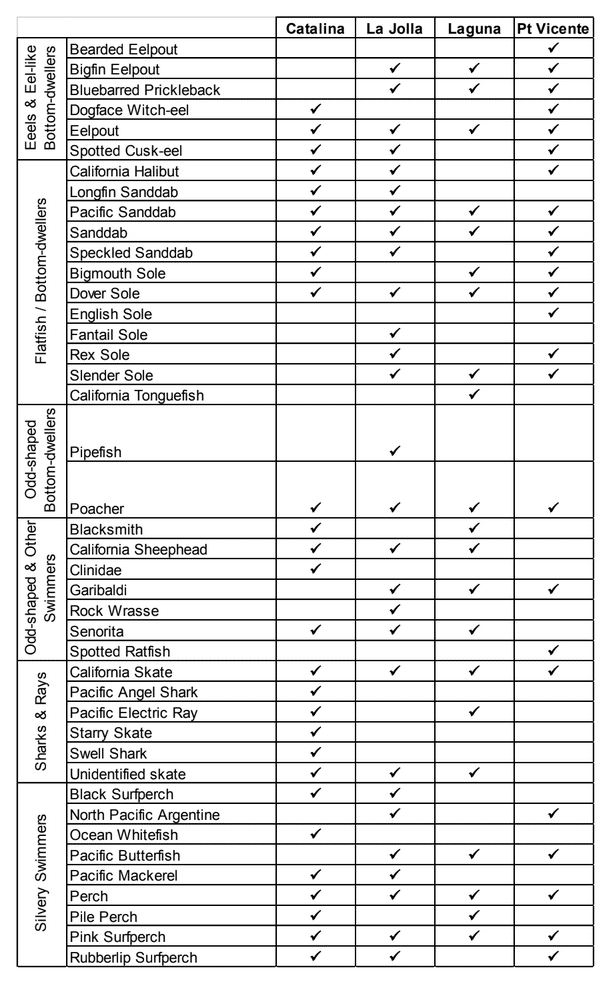
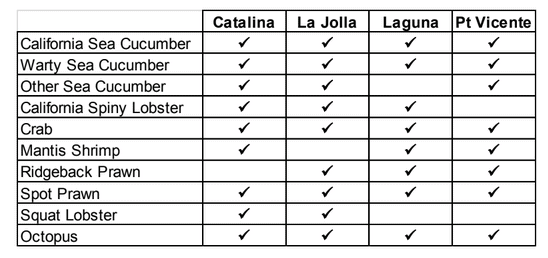
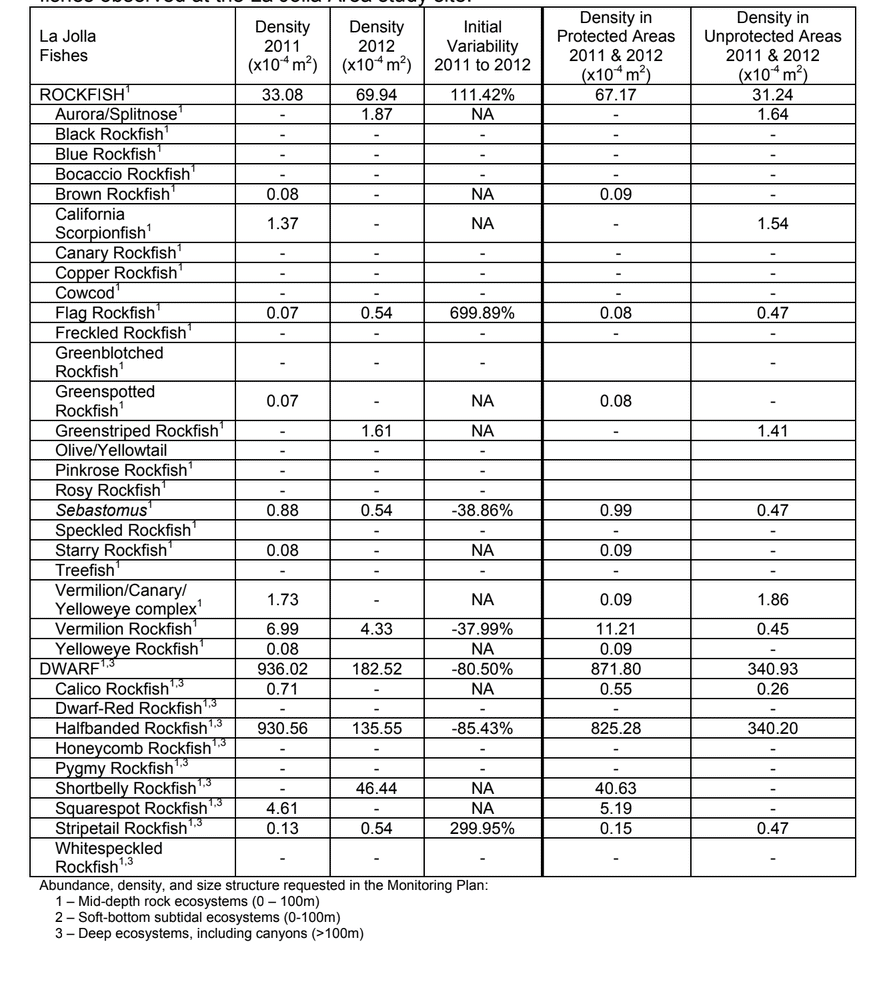
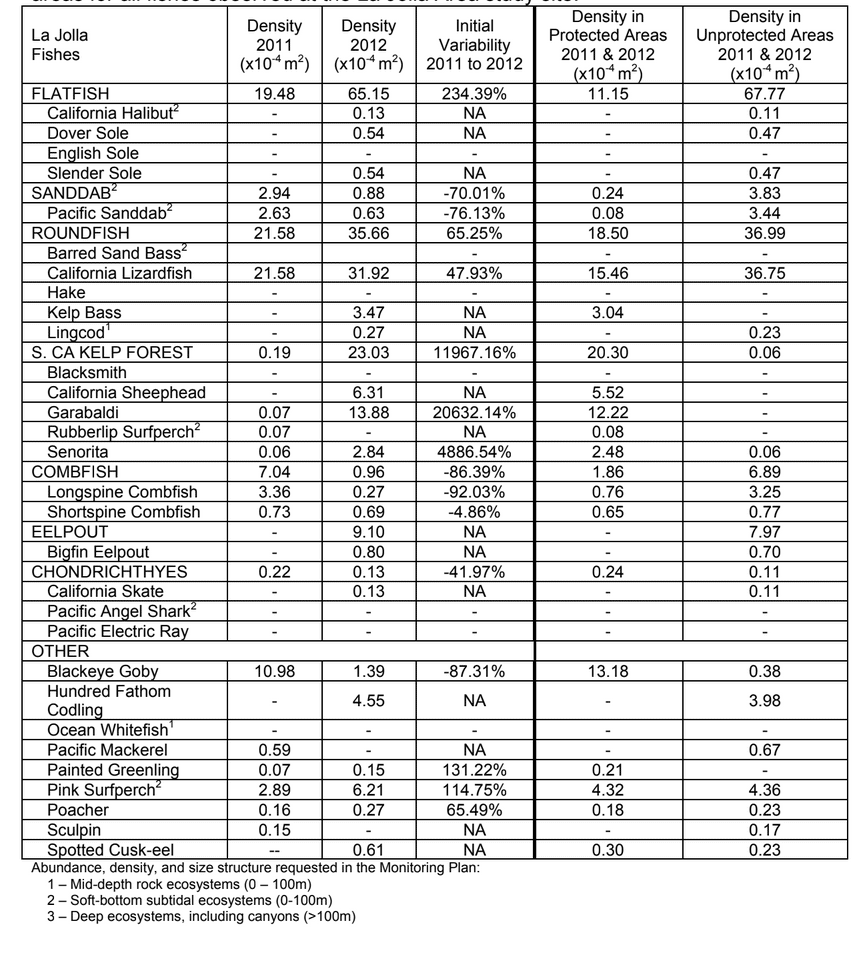
to rocky reefs, and the transitional areas in between. At the northernmost mainland site (Point Vicente), Halfbanded Rockfish were the most abundant of the 16,853 fish we observed. It is important to note that we were prevented from sampling the limited rocky reef areas along the mainland due to the significant entanglement hazards created by Giant Kelp (Macrocystis pyrifera) and lobster pots. In the south (La Jolla), which included both shelf sites as well as sites deep within the submarine canyons, Halfbanded Rockfish also dominated the 16,867 fish observed, despite very challenging sampling conditions. Indeed, to account for the great difficulties we encountered sampling the deep submarine canyons, we developed a new sampling protocol described below in the section on Analytical products derived from baseline data. Of the 15,837 fish observed at the Farnsworth Bank MPAs along the southwest coast of Catalina Island, where visibility was generally excellent, Blacksmith were the most numerous (n=3,458). We also observed thousands of invertebrates, both mobile and sessile, across the study area. Insofar as this project was dedicated to a baseline characterization in support of future monitoring efforts, we targeted as many fishes (ranging from species to morphological groups) listed in the South Coast Monitoring Plan as could be sampled effectively with an ROV. We sampled a total of 13 (76.5%) of the fishes and fish groupings (e.g. “Rockfishes”) included in the monitoring plan, under ecosystems surveyed by the ROV (Table 1). Further, we sampled a total of 71% of invertebrate species and groups described in the monitoring plan (Table 2). Suggestions for Future Monitoring – Anticipating the challenge of sustaining a long-term monitoring effort well beyond the baseline provided here, we propose the following list of species/taxonomic groups for inclusion in a video-based monitoring program. These species, including both fishes and invertebrates, are a) observed in numbers that are appropriate for a variety of statistical analyses and b) are capable of being identified with a high level of confidence from imagery alone.
Insofar as this project was dedicated to a baseline characterization in support of future monitoring efforts, we targeted as many fishes (ranging from species to morphological groups) listed in the South Coast Monitoring Plan as could be sampled effectively with an ROV. We sampled a total of 13 (76.5%) of the fishes and fish groupings (e.g. “Rockfishes”) included in the monitoring plan, under ecosystems surveyed by the ROV (Table 1). Further, we sampled a total of 71% of invertebrate species and groups described in the monitoring plan (Table 2). Suggestions for Future Monitoring – Anticipating the challenge of sustaining a long-term monitoring effort well beyond the baseline provided here, we propose the following list of species/taxonomic groups for inclusion in a video-based monitoring program. These species, including both fishes and invertebrates, are a) observed in numbers that are appropriate for a variety of statistical analyses and b) are capable of being identified with a high level of confidence from imagery alone.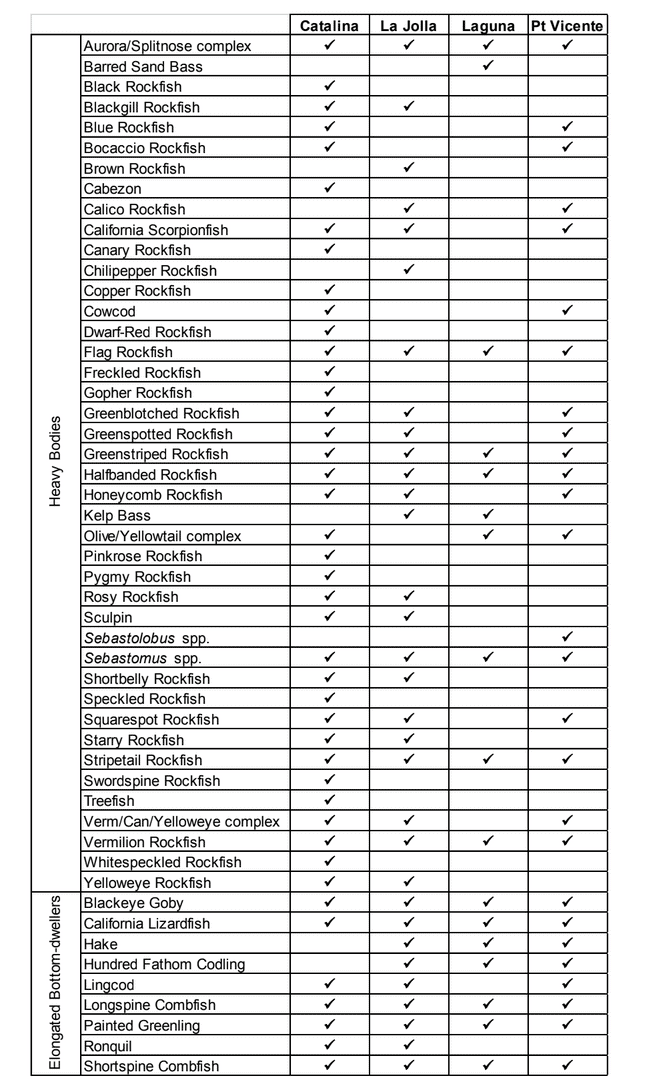
 estimated 29% (1840 km2) of state waters in southern California, yet they are sampled with far less frequency when compared to shallower waters due to the many challenges associated with sampling in deep water.
estimated 29% (1840 km2) of state waters in southern California, yet they are sampled with far less frequency when compared to shallower waters due to the many challenges associated with sampling in deep water.
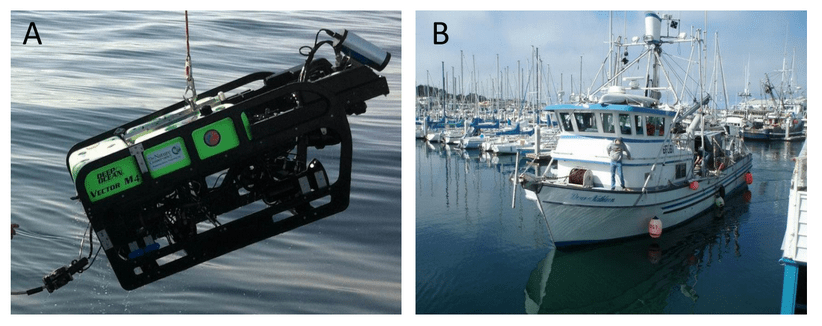
 video, forward-looking high-definition video, down-looking standard-definition video, digital still (forward or down positional), and rear facing video), two quartz halogen and HMI lights, paired forward- and down-looking sizing lasers (spaced at 10 cm), and a strobe for still photos. The ROV was also equipped with an altimeter, forward-facing multibeam sonar, CTD, and dissolved oxygen meter. The position of the ROV on the seafloor was maintained by the Trackpoint III® acoustic positioning system with the resulting coordinates logged into Hypack® navigational software. The ROV was ‘flown’ over
video, forward-looking high-definition video, down-looking standard-definition video, digital still (forward or down positional), and rear facing video), two quartz halogen and HMI lights, paired forward- and down-looking sizing lasers (spaced at 10 cm), and a strobe for still photos. The ROV was also equipped with an altimeter, forward-facing multibeam sonar, CTD, and dissolved oxygen meter. The position of the ROV on the seafloor was maintained by the Trackpoint III® acoustic positioning system with the resulting coordinates logged into Hypack® navigational software. The ROV was ‘flown’ over
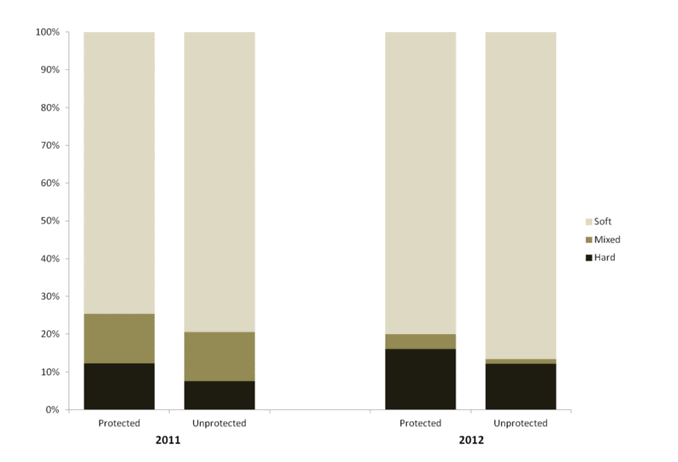
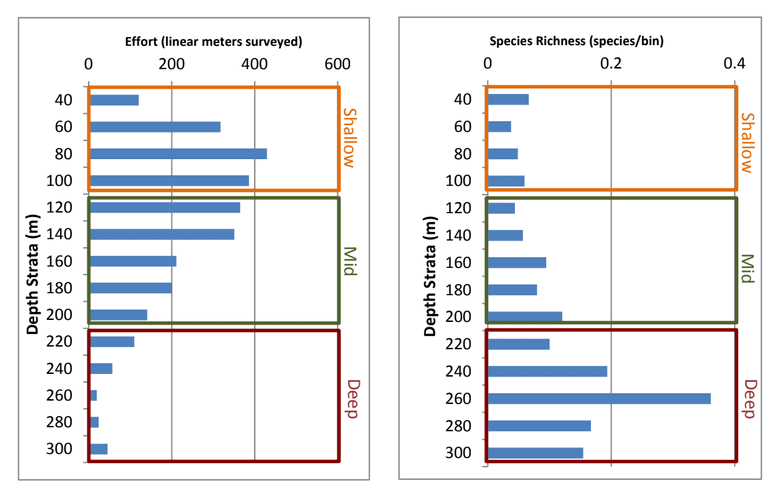
 research project, we sought to let the data drive the scale of the analyses rather than constraining the analyses to our a prior understanding of a particular species’ distribution. For on-going analyses of project data (summarized in a separate section below), sub-sampling of transect data occurred post hoc for selected species or taxonomic groups based on their distribution and considering the extent to which spatial autocorrelation influenced the data (Hallenbeck et al. 2012). Consequently,
research project, we sought to let the data drive the scale of the analyses rather than constraining the analyses to our a prior understanding of a particular species’ distribution. For on-going analyses of project data (summarized in a separate section below), sub-sampling of transect data occurred post hoc for selected species or taxonomic groups based on their distribution and considering the extent to which spatial autocorrelation influenced the data (Hallenbeck et al. 2012). Consequently,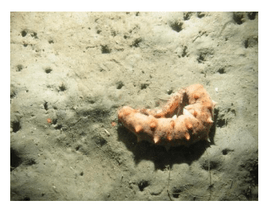 sessile invertebrate
sessile invertebrate future changes could be compared. As such, our sampling with the ROV at each location 2011 and 2012 was not intended to flesh out any differences between the two sampling periods. Further, given that our sampling was conducted essentially at the moment of designation for the MPAs, we were not focused on any “MPA effects” either. However, as questions inevitably arise about differences between sampling years, and between
future changes could be compared. As such, our sampling with the ROV at each location 2011 and 2012 was not intended to flesh out any differences between the two sampling periods. Further, given that our sampling was conducted essentially at the moment of designation for the MPAs, we were not focused on any “MPA effects” either. However, as questions inevitably arise about differences between sampling years, and between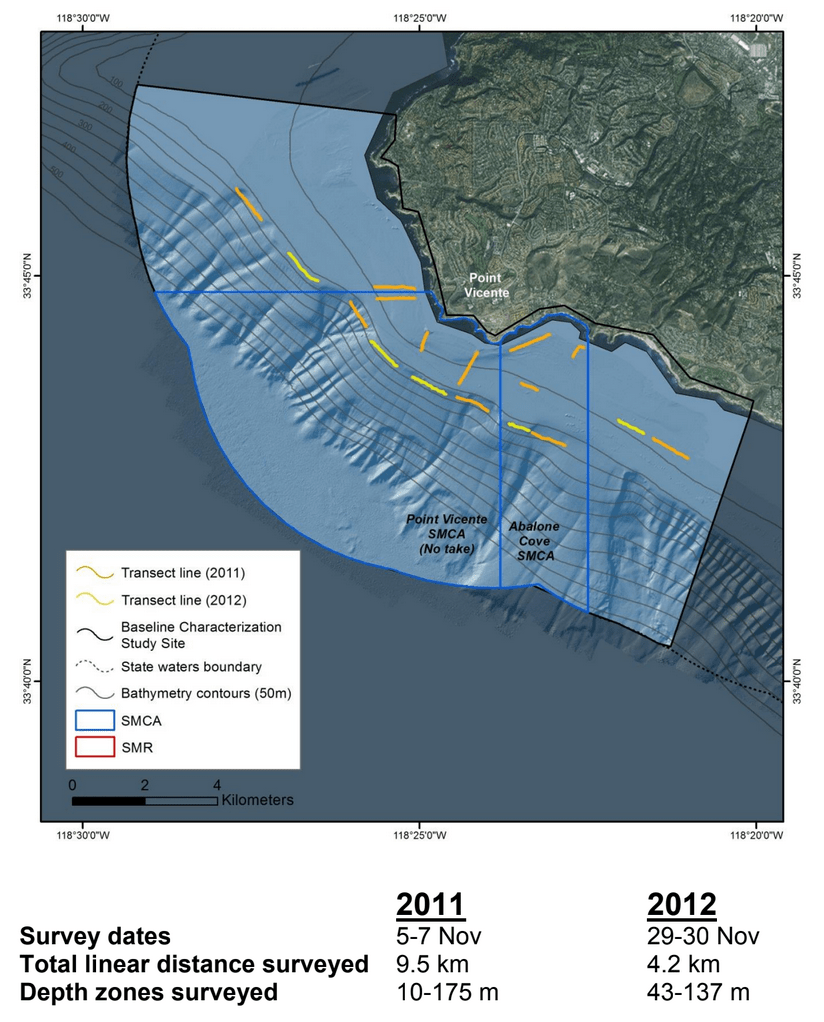



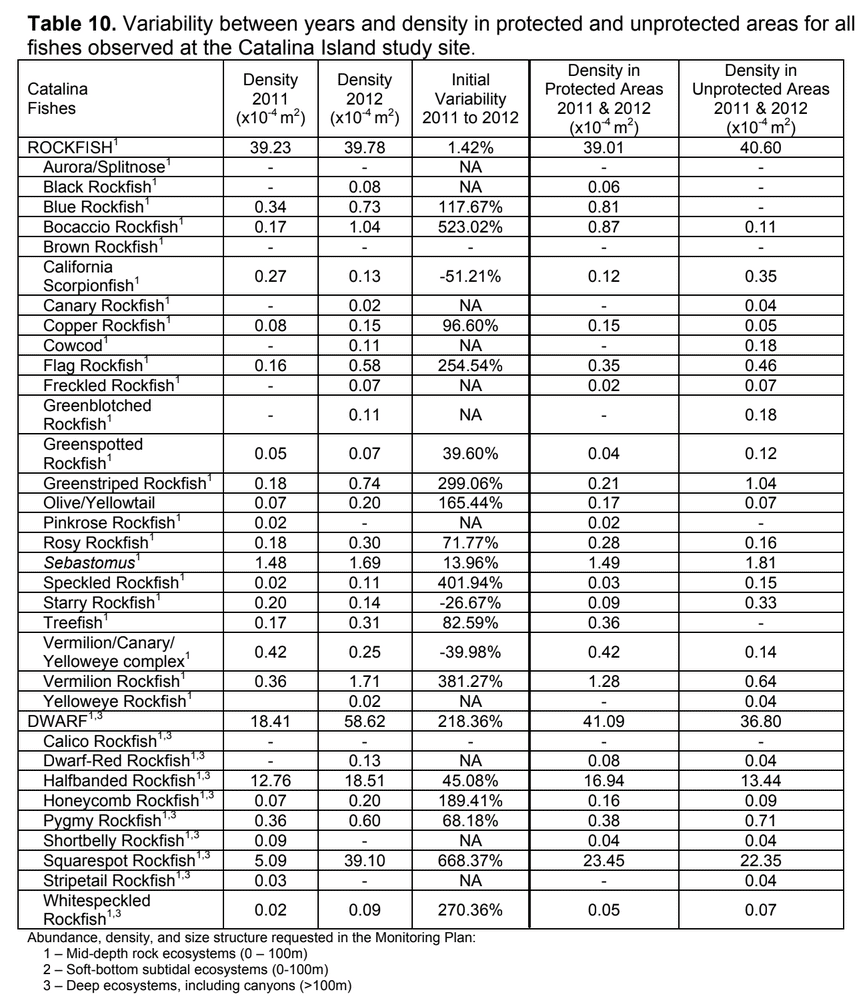
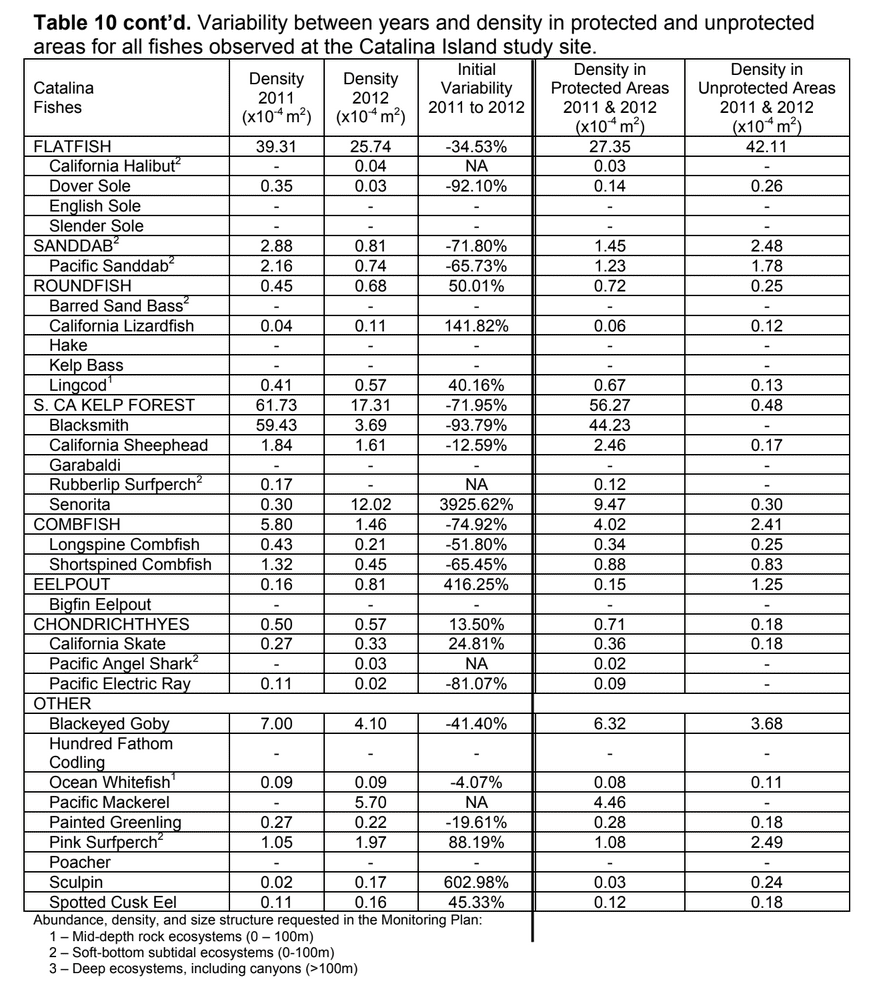
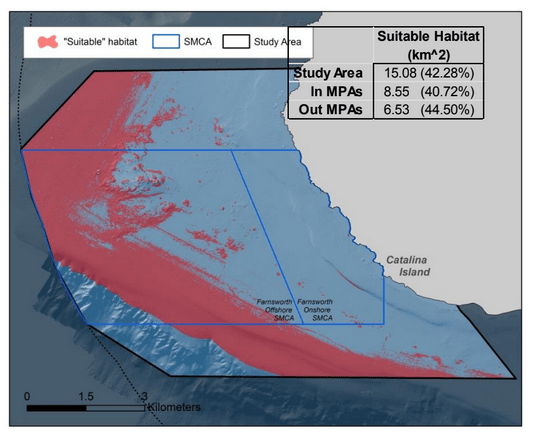
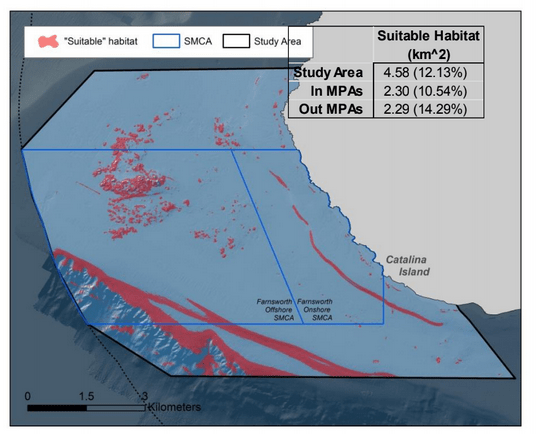
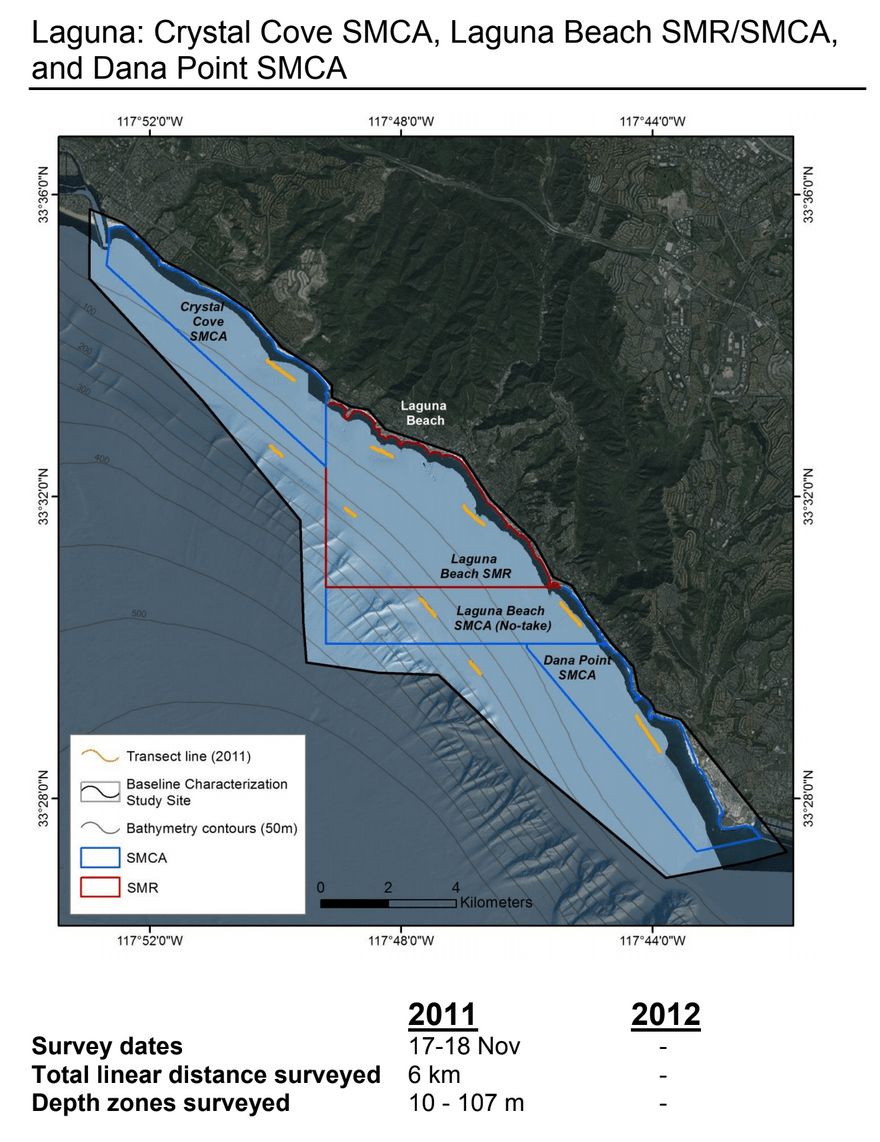
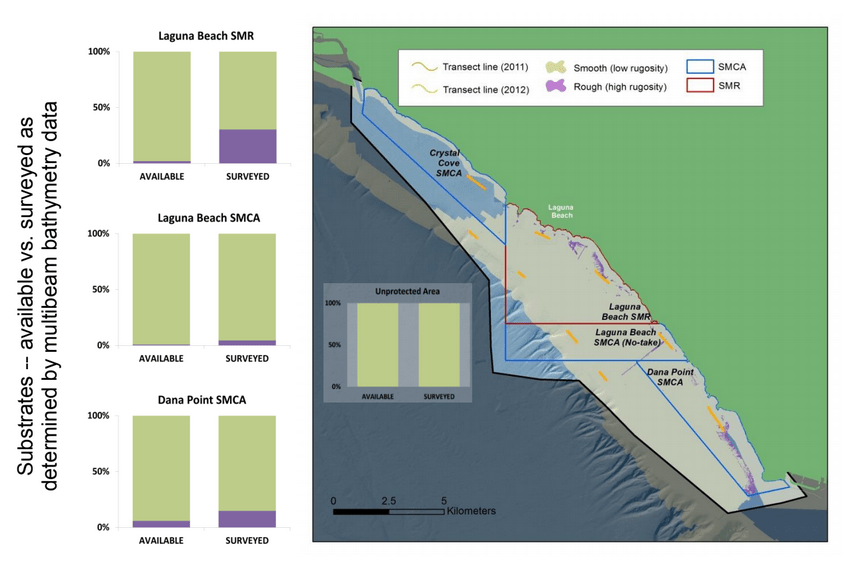


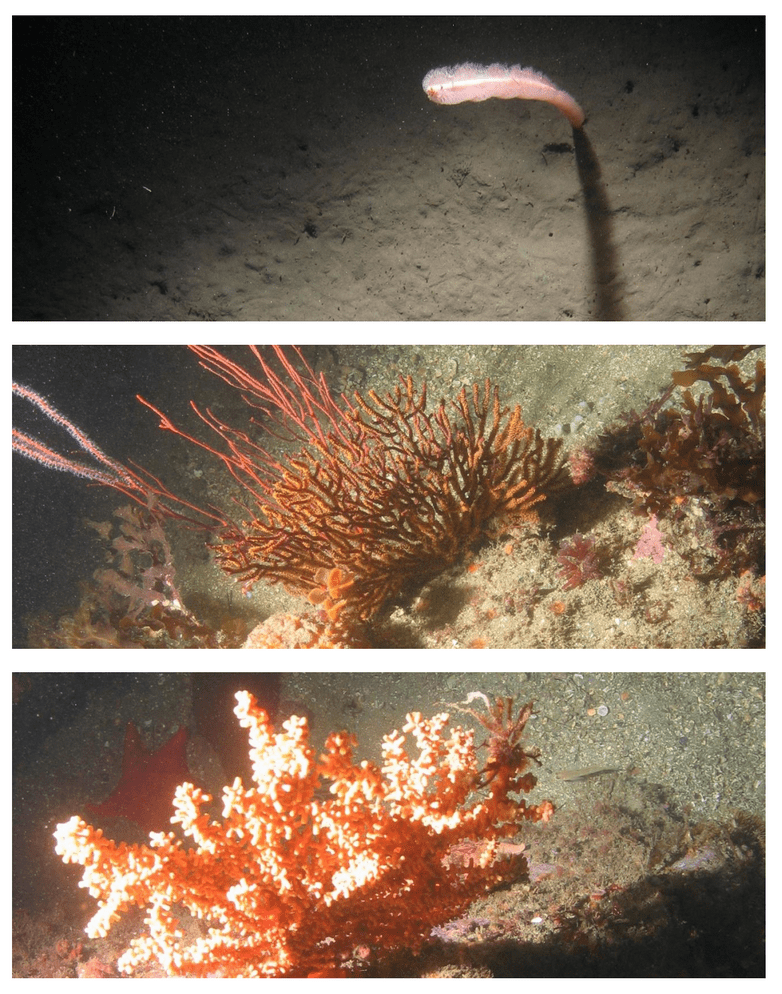
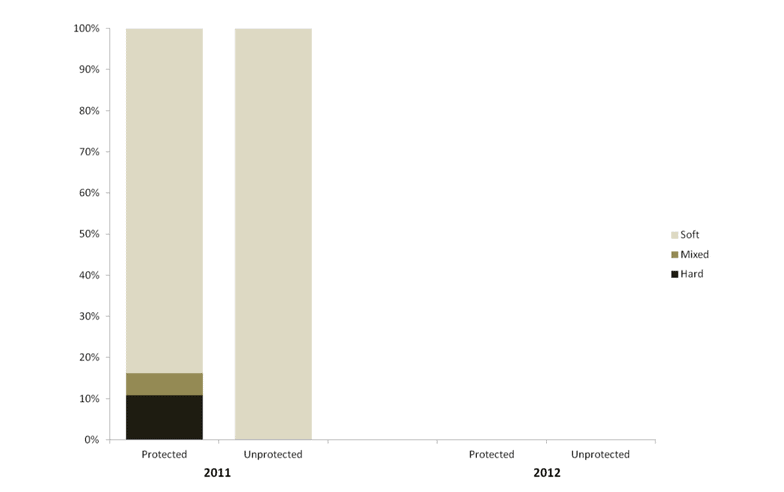
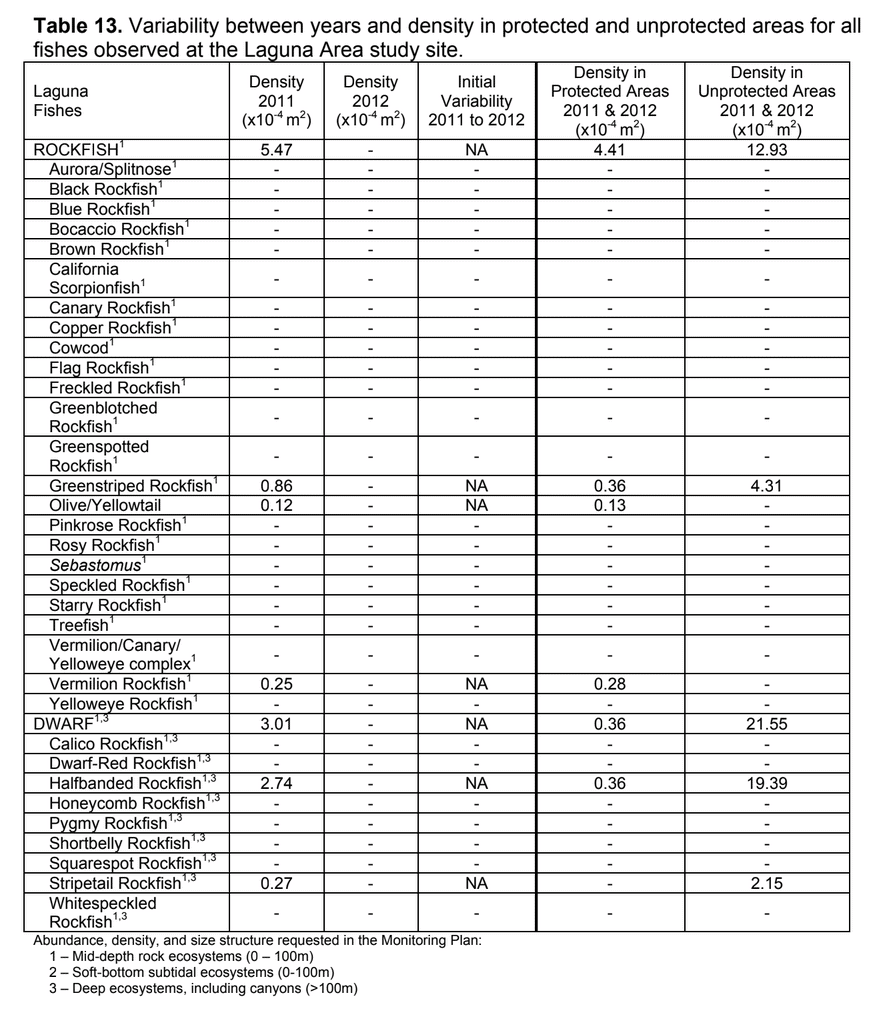
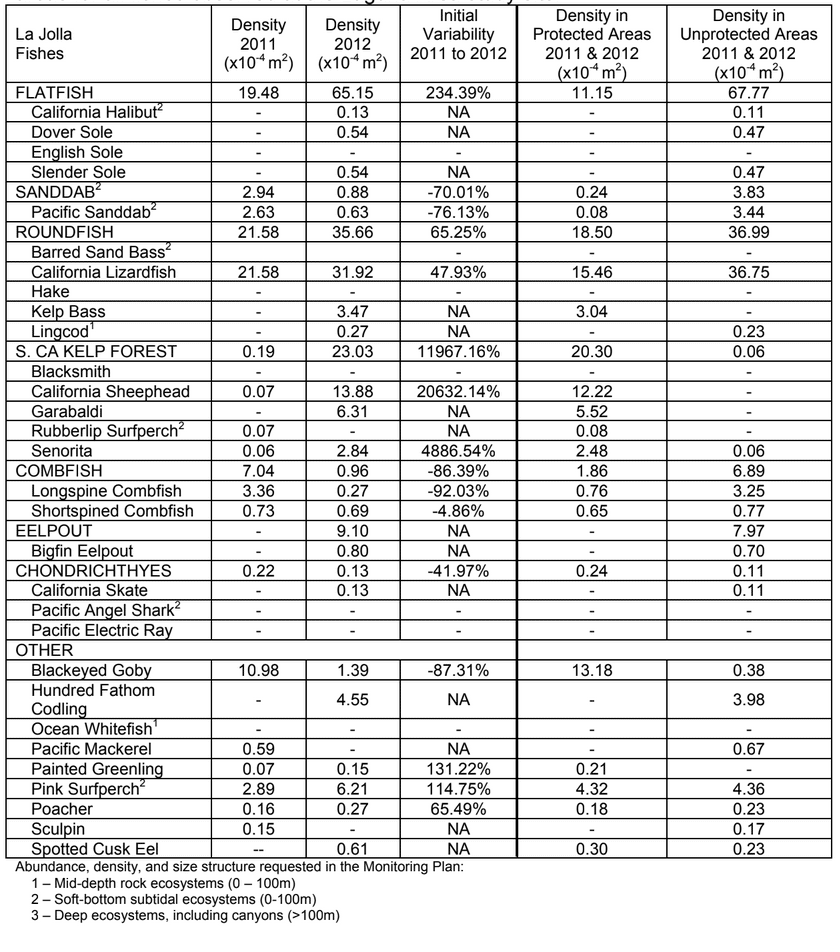
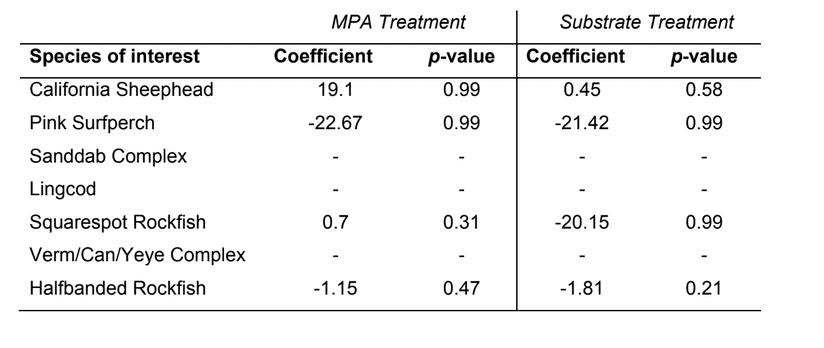
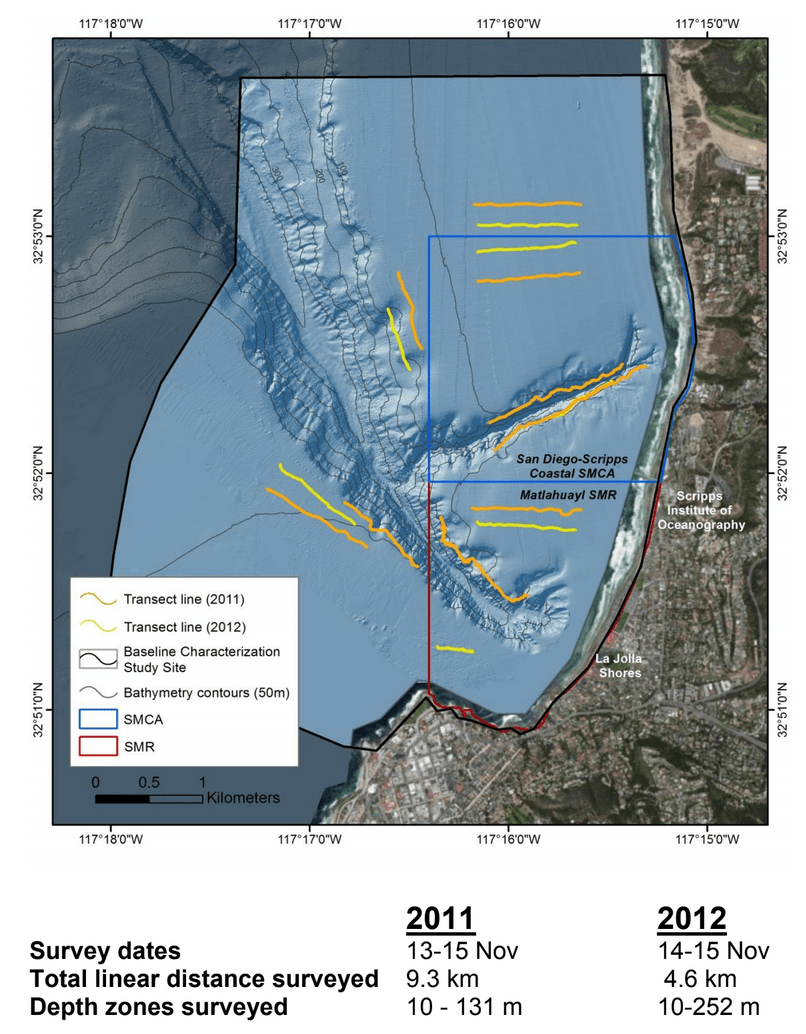
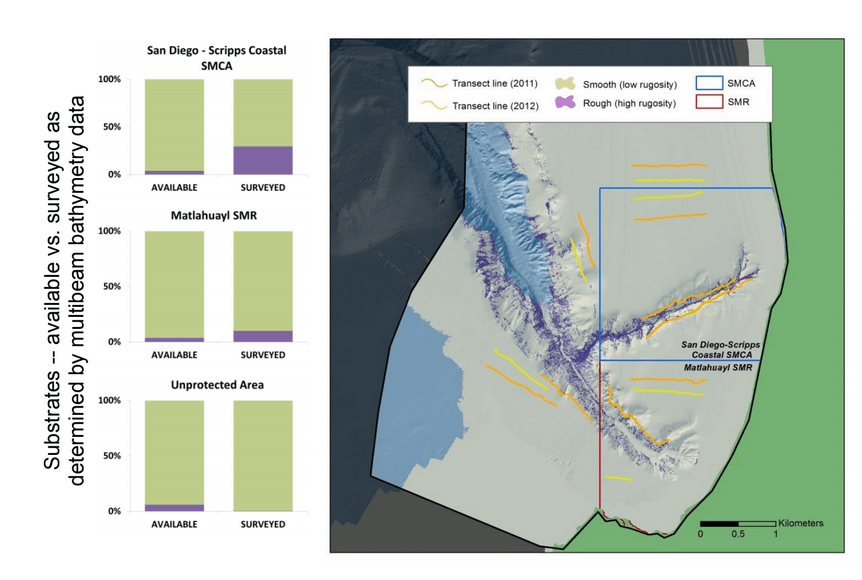



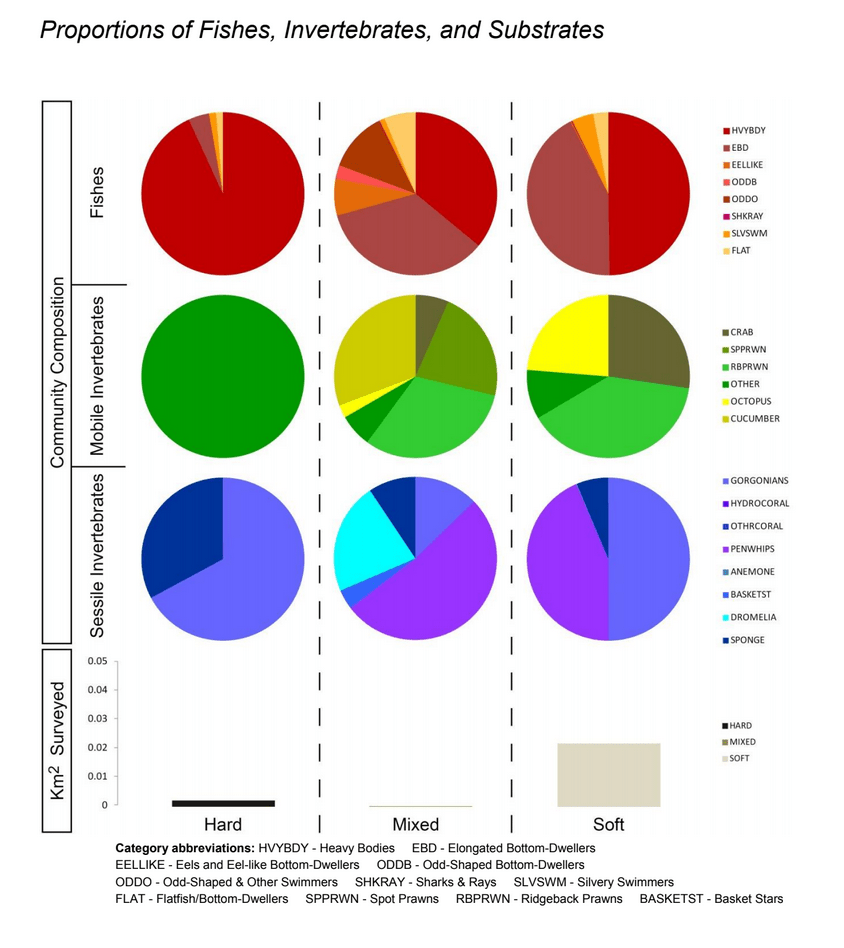
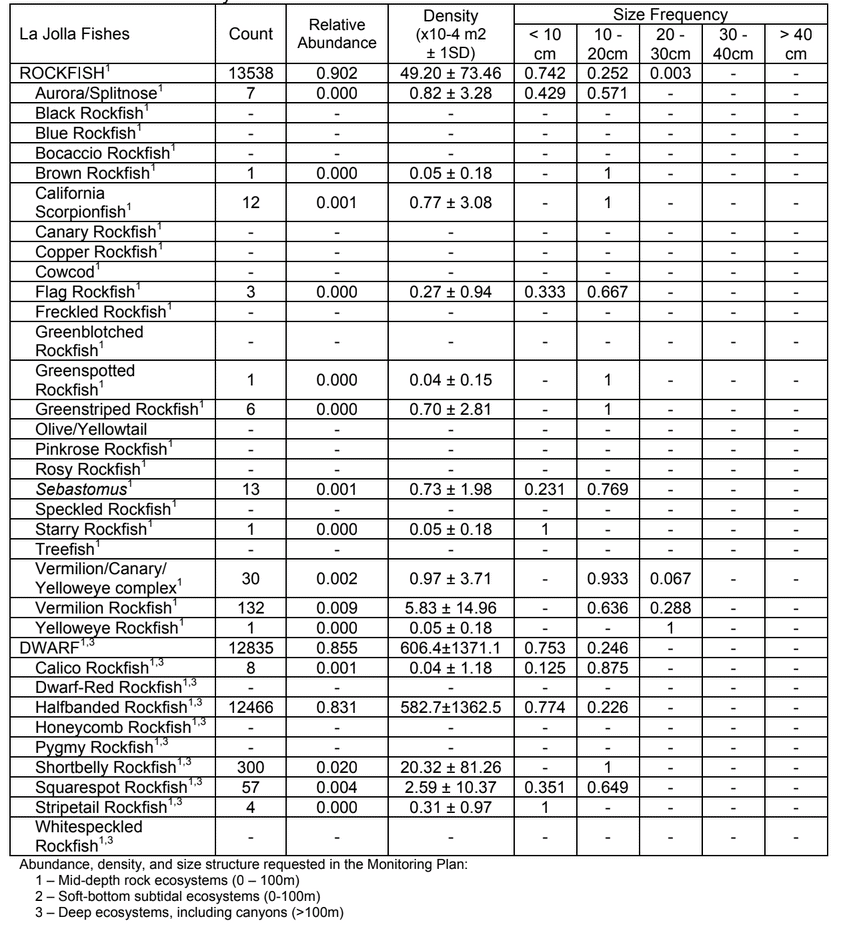
 Despite this importance, little is known about the structure, distribution, and
Despite this importance, little is known about the structure, distribution, and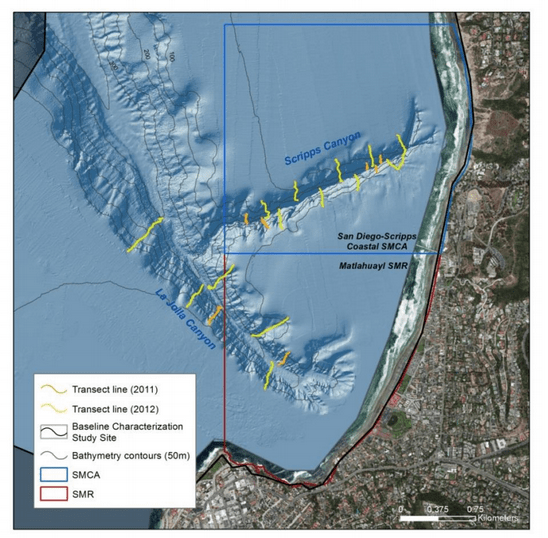
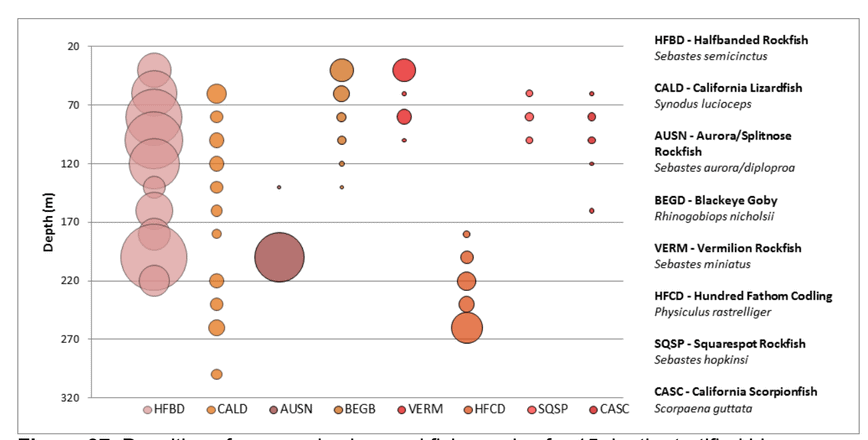
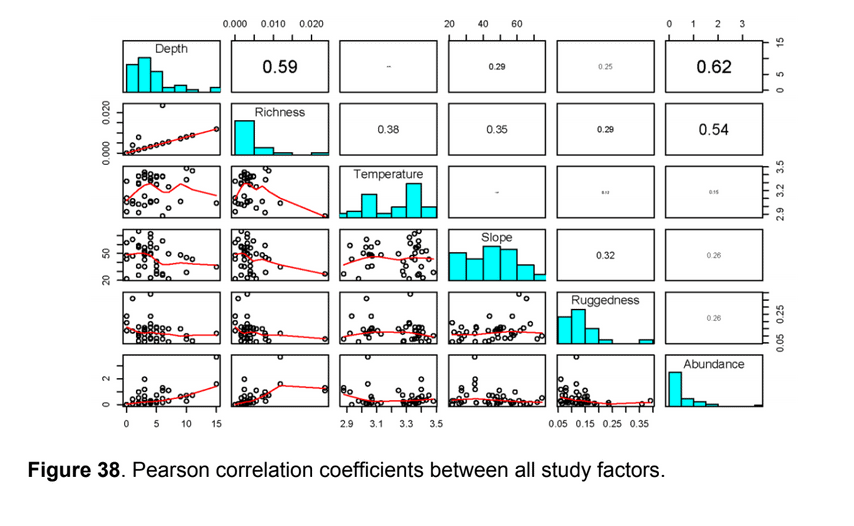
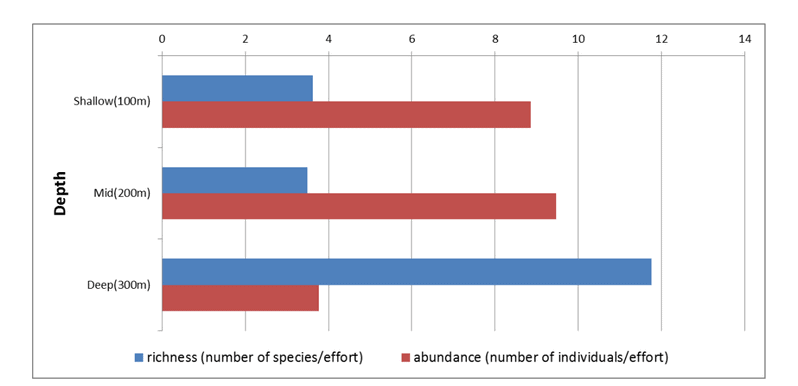
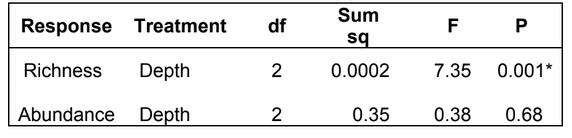
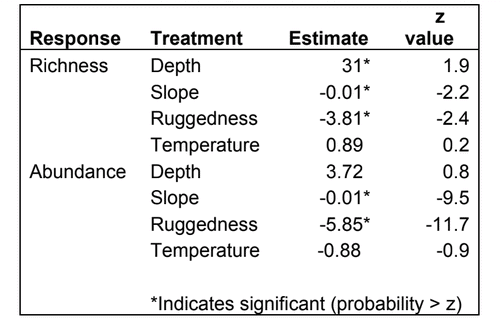
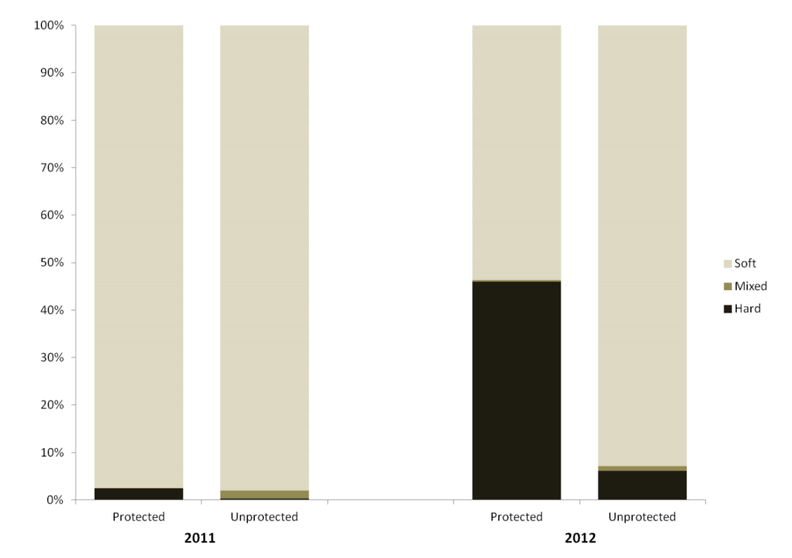
 state waters. Below are brief descriptions of two such on-going projects, one that describes the distributions of fishes and invertebrates with depth along the shelf and slope elsewhere throughout the sampled areas, and one that
state waters. Below are brief descriptions of two such on-going projects, one that describes the distributions of fishes and invertebrates with depth along the shelf and slope elsewhere throughout the sampled areas, and one that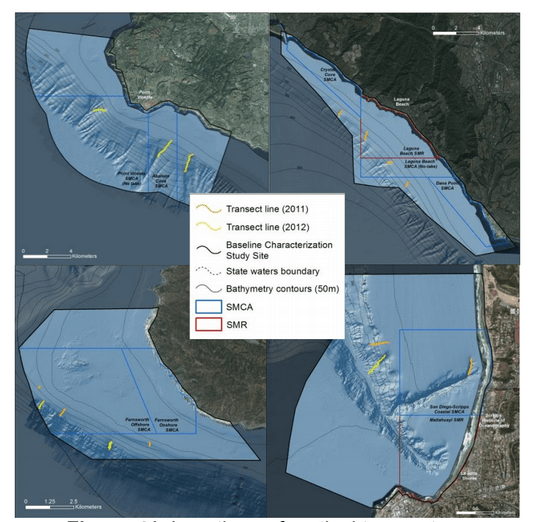
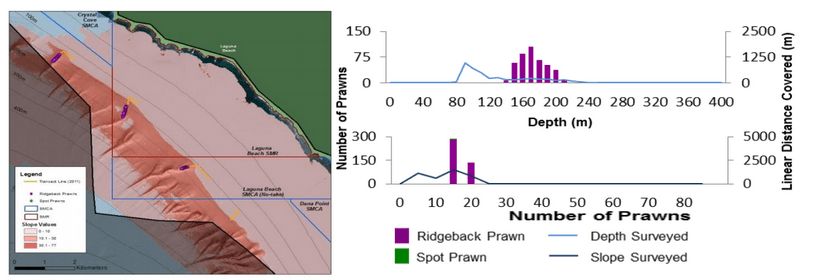
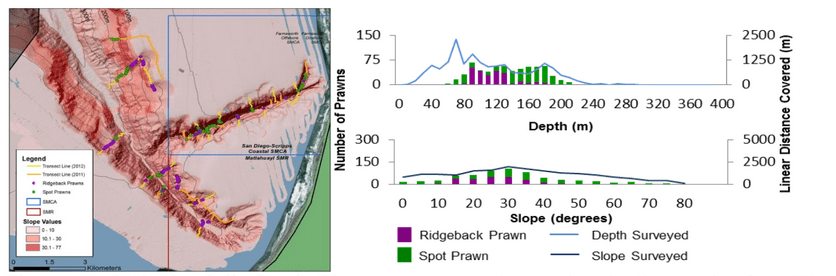
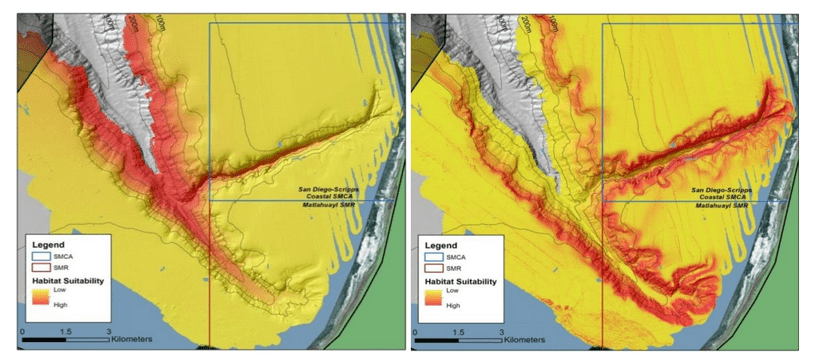
 (such as the areas sampled for this report) the likelihood of a strong citizen-based monitoring program coming to the fore is probably very low; working in the deep water is costly, including vessel support, vehicle support (ROV, submersible, camera sled), and the personnel necessary to operate both. And yet, despite the associated cost, the non-invasive sampling of marine ecosystems using imagery platforms has important advantages with so many marine populations at historically-low levels.
(such as the areas sampled for this report) the likelihood of a strong citizen-based monitoring program coming to the fore is probably very low; working in the deep water is costly, including vessel support, vehicle support (ROV, submersible, camera sled), and the personnel necessary to operate both. And yet, despite the associated cost, the non-invasive sampling of marine ecosystems using imagery platforms has important advantages with so many marine populations at historically-low levels. Below we provide a first pass at a group of species and species complexes, including fishes as well as mobile and sessile invertebrates, that are capable of being monitored in this way and were observed during the baseline characterization effort in the South Coast. While we expect that many scientists could reach agreement on some of the organisms on this list, it is also likely that much discussion could be engendered to flesh this group out further. What we provide here is intended as a point of departure for discussion as each of the MLPA regions moves beyond baseline characterization.
Below we provide a first pass at a group of species and species complexes, including fishes as well as mobile and sessile invertebrates, that are capable of being monitored in this way and were observed during the baseline characterization effort in the South Coast. While we expect that many scientists could reach agreement on some of the organisms on this list, it is also likely that much discussion could be engendered to flesh this group out further. What we provide here is intended as a point of departure for discussion as each of the MLPA regions moves beyond baseline characterization.